THE CHEMICAL NATURE OF ENZYMES
Evidence of protein nature of enzymes:
1. Enzymes are broken down by hydrolysis into amino acids.
2. Under the action of boiling and other factors enzymes are denatured and lose their catalytic activity.
3. The enzymes are extracted from solutions in the form of protein crystals.
4. Enzymes have highly specific effects.
The direct evidence of protein nature of enzymes is the first laboratory synthesis of the enzyme ribonuclease.
Some enzymes are simple, consisting only of the polypeptide chain: pepsin, trypsin, urease, ribonuclease, phosphatase, etc.
Most of the natural enzymes are conjugative proteins. Their protein part is called apoenzyme. Complex enzymes include non-protein components or cofactors.Enzymes can’t perform their catalytic function without cofactors.
Cofactorsare vitamins or compounds constructed with their participation (coenzyme A, NAD+, FAD), phosphoric esters of some monosaccharides, metals ions (Zn2+, Mg2+, Mn2+, Fe2+).
Coenzymeis non-protein factor, which is easily separated from the protein part or apoenzyme.
Prosthetic group is non-protein component which is covalently bounded to the protein chain. It is not separated by the isolation and purification of the enzyme.
Apoenzyme with a prosthetic group is holoenzyme. Only holoenzyme has catalytic activity.
Substrateis the substance that is converted by the enzyme action.
Active site is the specific site on the enzyme where substrate molecule is bonded and directly involved in catalysis. The active sites of enzymes are produced at the level of tertiary structure. In complex enzymes cofactors are also included in the active site. Cofactors of enzymes act as intermediate vectors of the atoms or groups.
In the active site there are two parts. The substrate (binding) center is a site that is responsible for adherence of the substrate. It is called the contact or "anchor" site of the enzyme. The catalytic center is responsible for the chemical conversion of the substrate. In the structure of the catalytic site of most enzymes there are amino acids such as serine, cysteine, histidine, tyrosine, lysine.
The substrate center can overlap (or match) with the catalytic center.
Allosteric center is site of the enzyme molecule outside the active center, which is able to bind by weaker types of bonds with a specific substance (ligand). Result of it is changing the tertiary and quaternary structure of the protein molecule. It leads to changing the configuration of the active site and changes the catalytic activity of the enzyme. This is allosteric regulation of catalytic activity of enzymes. Enzymes which catalytic activity is changed under the influence of allosteric effectors called allosteric enzymes.
Some of the enzymes are multifunctional. They have several enzymatic activities, but only a single polypeptide chain. This single chain is formed by several domains, and each is characterized by its own catalytic activity. For example, alcohol dehydrogenase not only catalyzes the oxidation of alcohols, but also the reaction of neutralization of xenobiotics.
Isozymes are multiple forms of the enzyme catalyzing the same reaction, but differ from each other in physical and chemical properties: the affinity to the substrate, activity and electrophoretic mobility.
A classic example is the enzyme lactate dehydrogenase, which accelerates the conversion of lactate to pyruvate and vice versa. Its molecule consists of four subunits of two types - H and M (from the English heart and muscle). This enzyme is due to different combinations of subunits can exist in five forms:
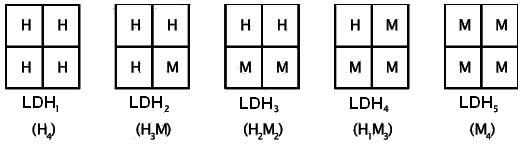
In norm each tissue is characterized by its own ratio of LDH forms (isozyme range). For example, in the heart muscle predominant H4, i.e. LDH1, and in skeletal muscle and liver M4 (LDH5). The study of appearance of LDH isoenzymes in blood serum gives an indication of the place of the pathological process and the extent of the affected organ or tissue.
A special group of enzymes are multimolecular enzyme complexes, composed of different enzymes catalyzing the consecutive stages of substrate conversion. The existence of such complexes increases chemical processes.
In cases where multienzyme complex serves a single, multi-step process of biochemical reactions, it is called metabolon. For example, metabolon of glycolysis, Krebs cycle, respiratory chain of mitochondria, etc.
Дата добавления: 2018-09-24; просмотров: 798;
