Amphoteric Nature of Water
Water has the ability to act as both an acid (proton donor) as well as a base (proton acceptor). It acts as a base to acids stronger than itself, and acts as an acid to bases stronger than itself. This amphoteric nature is clearly visible in the reaction given below, wherein water molecule reacts with acid as a base.
H2O (l) + HCl (aq) ⇌ H3O+ + Cl-
Water molecule also reacts with a strong base as an acid.
H2O (l) + NH3 (aq) ⇌ NH4+ + OH-
Another interesting property is that metals such as gold, silver, copper, tin, etc. do not react with water. Moreover, although the chemical properties of salt water differ from that of regular water, due to the different dissolved salts present in them, the chemical properties of distilled water (DW) are the same as those of normal water. DW will only differ in its physical properties.
Chemical Properties of Water
Water is called the 'universal solvent' because it dissolves more substances than any other liquid. This means that wherever water goes, either through the ground or through our bodies, it takes along valuable chemicals, minerals, and nutrients.
1) Solid electrolytes are composed of ions which are held together by electrostatic forces of attraction. When an electrolyte is dissolved in water, these forces are weakened and the electrolyte undergoes dissociation into ions. The ions are solvated. The process of splitting of the molecules into ions of an electrolyte is called dissociation (ionization).
HCl (g) + H2O (aq) = H+ (aq) + Cl-(aq)
NaOH (s) + H2O (aq) = Na+ (aq) + OH-(aq)
NaCl(s) + H2O (aq) = Na+ (aq) + Cl-(aq)
2) Reaction with metals:
a) Active metals (IA group) react vigorously with cold water and forming soluble bases (alkalis) at room temperature: 2Na (s) + 2H2O (aq) = 2NaOH (aq) + H2 (gas)
b) Metals from magnesium to iron in the activity series of metals, react with steam (but not H2O) to form the metal oxide and hydrogen gas:
Zn (s) + H2O (aq) = ZnO (s) + H2 (gas)
c) passive metals (Bi, Hg, Cu. Ag, Au, Pt) do not react with water and steam.
3) Water reacts with anhydrides (acidic oxides) and forming acids:
SO3 (g) + H2O (aq) = H2SO4 (aq)
4) Hydrolysis of salts: Na2S + 2H2O = 2NaOH + H2S
5) Electrolysis (this redox process, which takes place at the electrodes during then electric current is passed through the melt or solution of electrolytes and it is another way to get pure metals, nonmetals and acids) occur in aqueous solution:
2CuSO4 + 2H2O = 2Cu + O2 + 2H2SO4
Water Hardness
The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Hard water is high in dissolved minerals, both calcium and magnesium. You may have felt the effects of hard water, literally, the last time you washed your hands. Depending on the hardness of your water, after using soap to wash you may have felt like there was a film of residue left on your hands. In hard water, soap reacts with the calcium (which is relatively high in hard water) to form "soap scum". When using hard water, more soap or detergent is needed to get things clean, be it your hands, hair, or your laundry.
Have you done a load of dishes in the dishwasher, taken out the glasses, and noticed spots or film on them? This is more hard-water residue—not dangerous, but unsightly. Many industrial and domestic water users are concerned about the hardness of their water. When hard water is heated, such as in a home water heater, solid deposits of calcium carbonate can form. This scale can reduce the life of equipment, raise the costs of heating the water, lower the efficiency of electric water heaters, and clog pipes. And, yes, mineral buildup will occur in your home coffee maker too, which is why some people occassionally run vinegar (an acid) through the pot.
Hardness is caused by compounds of calcium and magnesium, and by a variety of other metals. General guidelines for classification of waters are:
· 0 to 60 mg/L (milligrams per liter) as calcium carbonate is classified as soft;
· 61 to 120 mg/L CaCO3 as moderately hard;
· 121 to 180 mg/L CaCO3 as hard;
· and more than 180 mg/L CaCO3 as very hard water.
A film left on a glass after dishwashing, the result of dissolved calcium and magnesium. Hard water can leave a film on glasses coming out of the dishwasher.
These "hardness ions" cause two major kinds of problems. First, the metal cations react with soaps, causing them to form an unsightly precipitate— the familiar "bathtub ring".
More seriously, the calcium and magnesium carbonates tend to precipitate out as adherent solids on the surfaces of pipes and especially on the hot heat exchanger surfaces of boilers. The resulting scale buildup can impede water flow in pipes. In boilers, the deposits act as thermal insulation that impedes the flow of heat into the water; this not only reduces heating efficiency, but allows the metal to overheat, which in pressurized systems can lead to catastrophic failure.
But hard water can have some benefits, too. Humans need minerals to stay healthy, and the National Research Council (National Academy of Sciences) states that hard drinking water generally contributes a small amount toward total calcium and magnesium human dietary needs.
Types of water hardness
· Temporary hardness: This refers to hardness whose effects can be removed by boiling the water in an open container. Such waters have usually percolated though limestone formations and contain bicarbonate HCO3– along with small amounts of carbonate CO32– as the principal negative ions. Boiling the water promotes the reaction
2 HCO3– → CO32– + CO2
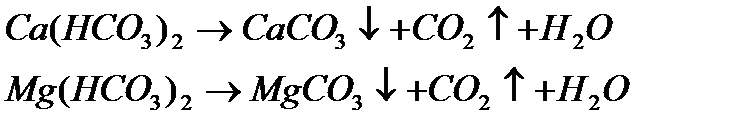 by driving off the carbon dioxide gas. The CO32– reacts with Ca2+ or Mg2+ ions, to form insoluble calcium and magnesium carbonates which precipitate out. By tying up the metal ions in this way, the amounts available to form soap scum are greatly reduced.
by driving off the carbon dioxide gas. The CO32– reacts with Ca2+ or Mg2+ ions, to form insoluble calcium and magnesium carbonates which precipitate out. By tying up the metal ions in this way, the amounts available to form soap scum are greatly reduced.
· Permanent hardness: Waters than contain other anions such as chloride or sulfate (CaCl2, MgCl2, CaSO4, MgSO4) cannot be remediated by boiling, and are said to be "permanently" hard. The only practical treatment is to remove all the ions, normally by the method described below.
Water is softened on a large scale by the addition of just enough lime to precipitate the calcium as carbonate and the magnesium as hydroxide, whereupon sodium carbonate is added to remove the remaining calcium salts.
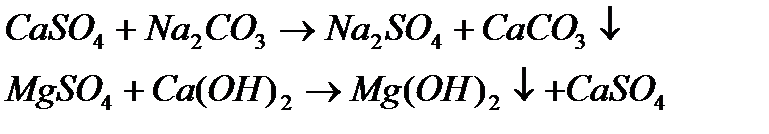
In areas where the water is hard, home water softeners are used, making use of the properties of natural or artificial zeolite minerals.
Conventional water softening
Most conventional water-softening devices depend on a process known as ion-exchange in which "hardness" ions trade places with sodium and chloride ions that are loosely bound to an ion-exchange resin or a zeolite (many zeolite minerals occur in nature, but specialized ones are often made artificially.)
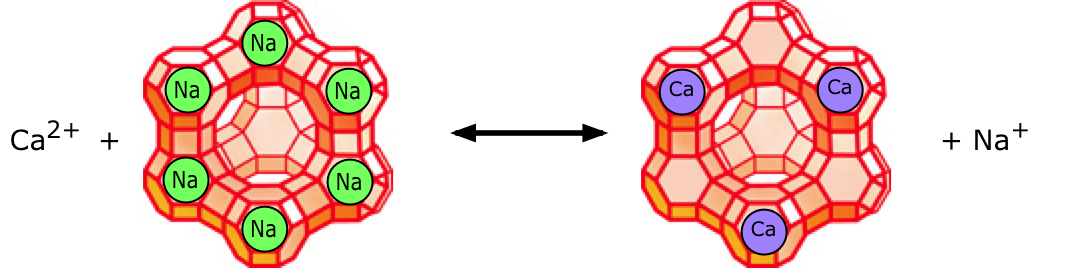
The illustration depicts a negatively-charged zeolite to which [positive] sodium ions are attached. Calcium or magnesium ions in the water displace sodium ions, which are released into the water. In a similar way, positively-charged zeolites bind negatively-charged chloride ions (Cl–), which get displaced by bicarbonate ions in the water. As the zeolites become converted to their Ca2+ and HCO3– forms they gradually lose their effectiveness and must be regenerated. This is accomplished by passing a concentrated brine solution though them, causing the above reaction to be reversed. Herein lies one of the drawbacks of this process: most of the salt employed in the regeneration process gets flushed out of the system and and is usually released into the soil or drainage system – something that can have damaging consequences to the environment, especially in arid regions. For this reason, many jurisdications prohibit such release, and require users to dispose of the spent brine at an approved site or to use a commercial service company.
Desalination
Desalination is the process of removing salt from sea-water to provide essential water for drinking, irrigation, and industry, especially in desert regions or areas where freshwater is scarce. In the almost 4,000 desalination plants worldwide, most desalination takes place through two methods: distillation and reverse osmosis:
· At its simplest, distillation consists of boiling seawater to separate it from dissolved salt. Once the seawater boils, water vapor rises, leaving the salt on the bottom of the tank. The water vapor is then transferred to a separate, cooler tank where it condenses as pure liquid water. Heat for distillation usually comes from burning fossil fuels (oil and coal). Distillation is widely used in the Middle East, where fossil fuel is plentiful but freshwater is scarce.
· Reverse osmosis uses high pressure to force pure water out of saltwater. Pressures up to 60 atmospheres (800 to 1,200 pounds per square inch) are applied to saltwater, forcing it through a special membrane that allows only pure water to flow through, trapping the salt on the other side. Reverse osmosis is widely used to desalinate brackish water, which is less salty than seawater and therefore requires pressures only about one-half as great.
Drinking water
Our ordinary drinking water, by contrast, is never chemically pure, especially if it has been in contact with sediments. Groundwaters (from springs or wells) always contain ions of calcium and magnesium, and often iron and manganese as well; the positive charges of these ions are balanced by the negative ions carbonate/bicarbonate, and occasionally some chloride and sulfate. Groundwaters in some regions contain unacceptably high concentrations of naturally-occuring toxic elements such as selenium and arsenic.
One might think that rain or snow would be exempt from contamination, but when water vapor condenses out of the atmosphere it always does so on a particle of dust which releases substances into the water, and even the purest air contains carbon dioxide which dissolves to form carbonic acid. Except in highly polluted atmospheres, the impurities picked up by snow and rain are too minute to be of concern.
Various governments have established upper limits on the amounts of contaminants allowable in drinking water; the best known of these are the U.S. EPA Drinking Water Standards.
What kind of water is most healthy to drink?
I am not aware of any evidence indicating that any one type of water (including highly "pure" water) is more beneficial to health than any other, as long as the water is pathogen-free and meets accepted standards such as those mentioned above. For those who are sensitive to residual chlorine or still have concerns, a good activated-carbon filter is usually satisfactory. More extreme measures such as reverse-osmosis or distillation are only justified in demonstrably extreme situations.
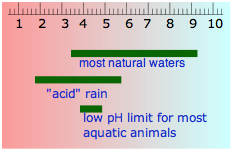 "Pure" rainwater always contains some dissolved carbon dioxide which makes it slightly acidic. When this water comes into contact with sediments, it tends to dissolve them, and in the process becomes alkaline. The pH of drinking water can vary from around 5 to 9, and it has no effect on one's health. The idea that alkaline water is better to drink than acidic water is widely promoted by alternative-health hucksters who market worthless "water ionizer" machines for this purpose. Acidic water is sometimes described by engineers as "aggressive"; this refers to its tendency to corrode metal distribution pipes, but in this sense it is no more active than the hydrochloric acid already present in your gastric fluid!
"Pure" rainwater always contains some dissolved carbon dioxide which makes it slightly acidic. When this water comes into contact with sediments, it tends to dissolve them, and in the process becomes alkaline. The pH of drinking water can vary from around 5 to 9, and it has no effect on one's health. The idea that alkaline water is better to drink than acidic water is widely promoted by alternative-health hucksters who market worthless "water ionizer" machines for this purpose. Acidic water is sometimes described by engineers as "aggressive"; this refers to its tendency to corrode metal distribution pipes, but in this sense it is no more active than the hydrochloric acid already present in your gastric fluid!
Ion-free water
One occasionally hears that mineral-free water, and especially distilled water, are unhealthy because they "leach out" required minerals from the body. There is no truth to this; the fact is that mineral ions do not pass through cell walls by ordinary osmotic diffusion, but rather are actively transported by metabolic processes. An extensive 2008 study failed to confirm earlier reports that low calcium/magnesium in drinking water correlates with cardiovascular disease. Any well-balanced diet should supply all the mineral substances we need.
It is well known that people who are engaged in heavy physical activity or are in a very hot environment should avoid drinking large quantities of even ordinary water. In order to prevent serious electrolyte imbalance problems, it is necessary to make up for the salts lost through perspiration. This can be accomplished by ingestion of salted foods or beverages (including "sports beverages"), or salt tablets.
Дата добавления: 2018-11-25; просмотров: 611;
