Chemical Substances
In chemistry, a chemical substance is a form of matter that has constant chemical composition and characteristic properties. It cannot be separated into components without breaking chemical bonds. Chemical substances can be solids, liquids, gases, or plasma. Changes in temperature or pressure can cause substances to shift between the different phases of matter.
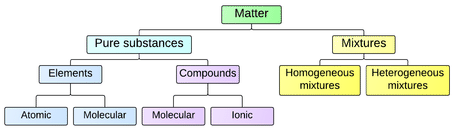
Elements.A chemical element is a pure substance that consists of one type of atom. Each atom has an atomic number, which represents the number of protons that are in the nucleus of a single atom of that element. The periodic table of elements is ordered by ascending atomic number. The chemical elements are divided into the metals, the metalloids, and the non-metals. Metals, typically found on the left side of the periodic table, are:
· often conductive to electricity
· malleable
· shiny
· sometimes magnetic.
Aluminum, iron, copper, gold, mercury and lead are metals.
In contrast, non-metals, found on the right side of the periodic table (to the right of the staircase), are:
· typically not conductive
· not malleable
· dull (not shiny)
· not magnetic.
Examples of elemental non-metals include carbon and oxygen.
Metalloids have some characteristics of metals and some characteristics of non-metals. Silicon and arsenic are metalloids.
As of November, 2011, 118 elements have been identified (the most recently identified was ununseptium, in 2010). Of these 118 known elements, only the first 98 are known to occur naturally on Earth. The elements that do not occur naturally on Earth are the synthetic products of man-made nuclear reactions. 80 of the 98 naturally-occurring elements are stable; the rest are radioactive, which means they decay into lighter elements over timescales ranging from fractions of a second to billions of years.
Hydrogen and helium are by far the most abundant elements in the universe. However, iron is the most abundant element (by mass) in the composition of the Earth, and oxygen is the most common element in the layer that is the Earth's crust.
Although all known chemical matter is composed of these elements, chemical matter itself constitutes only about 15% of the matter in the universe. The remainder is dark matter, a mysterious substance that is not composed of chemical elements. Dark matter lacks protons, neutrons, or electrons.
There are many substances that exist as two or more atoms connected together so strongly that they behave as a single particle. These multiatom combinations are called molecules. A molecule is the smallest part of a substance that has the physical and chemical properties of that substance.
Thus, molecules are made up of atoms that are held together by chemical bonds. These bonds form as a result of the sharing or exchange of electrons among atoms. There are two main types and some secondary types of chemical bonds:
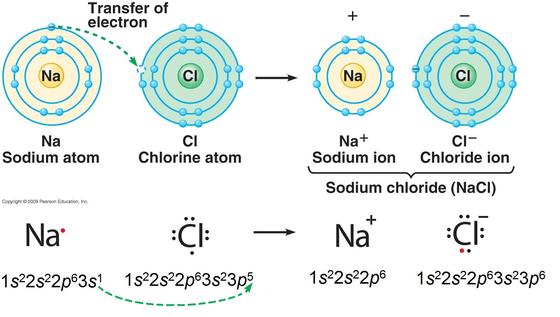
1) Sometimes atoms gain or lose electrons. Ionic bond involves a transfer of an electron, so one atom gains an electron while one atom loses an electron. Because opposite charges attract, the atoms bond together to form a molecule. The atom then loses or gains a "negative" charge. These atoms are then called ions.
Positive Ion- Occurs when an atom loses an electron (negative charge) it has more protons than electrons.
Negative Ion - Occurs when an atom gains an electron (negative charge) it will have more electrons than protons.
The following image shows Na losing an electron and Cl gaining an electron. Thus the Na atom becomes cation Na+, and the Cl atom becomes anion Cl-.
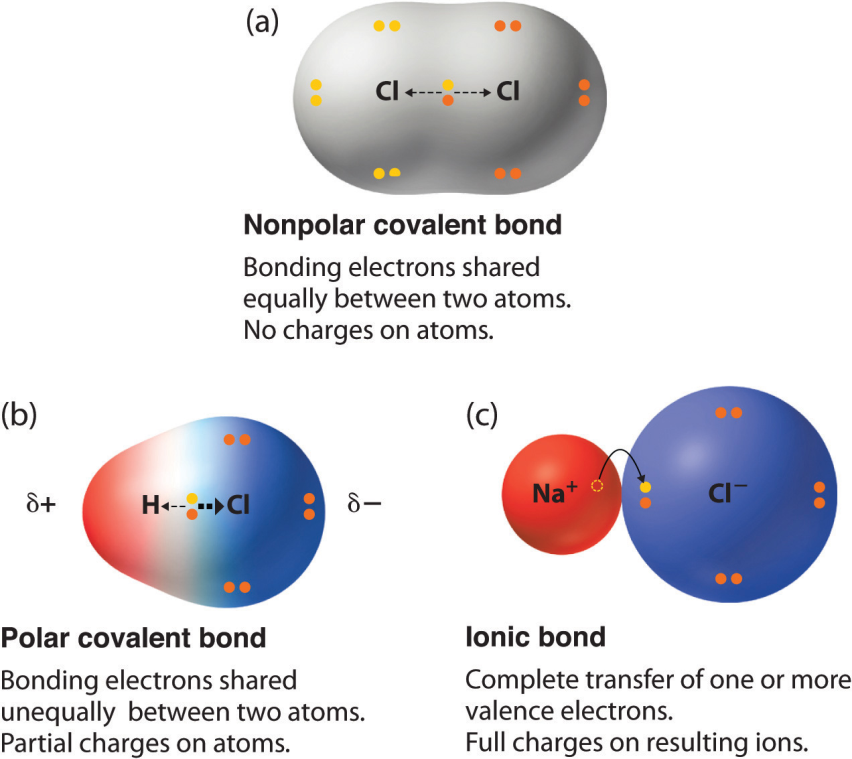
2) Covalent bond. The most common bond in organic molecules, a covalent bond involves the sharing of electrons between two atoms. The pair of shared electrons forms a new orbit that extends around the nuclei of both atoms, producing a molecule. There are two secondary types of covalent bonds that are relevant to Chemistry — polar and nonpolar bonds.
a) Polar bond. Two atoms connected by a covalent bond may exert different attractions for the electrons in the bond, producing an unevenly distributed charge. The result is known as a polar bond, an intermediate case between ionic and covalent bonding, with one end of the molecule slightly negatively charged and the other end slightly positively charged.
These slight imbalances in charge distribution are indicated in the figure by lowercase delta symbols with a charge superscript (+ or –). Although the resulting molecule is neutral, at close distances the uneven charge distribution can be important. Water is an example of a polar molecule; the oxygen end has a slight positive charge whereas the hydrogen ends are slightly negative. Polarity explains why some substances dissolve readily in water and others do not.
b) Nonpolar covalent bonds form between two atoms of the same element, or between atoms of different elements that share electrons more or less equally. For example, molecular oxygen O2, is nonpolar because the electrons are equally shared between the two oxygen atoms.
3) Hydrogen bond. In a polar covalent bond containing hydrogen (e.g., an O-H bond in a water molecule), the hydrogen will have a slight positive charge because the bond electrons are pulled more strongly toward the other element. Because of this slight positive charge, the hydrogen will be attracted to any neighboring negative charges. This interaction is called a hydrogen bond.
Hydrogen bonds are common, and water molecules in particular form lots of them. Individual hydrogen bonds are weak and easily broken, but many hydrogen bonds together can be very strong.
4) The force that binds together the atoms of metals is called metallic bond. A metallic bond results from the sharing of a variable number of electrons by a variable number of atoms. To account for the bonding in metals, Lorentz proposed a model known as electron gas model or electron sea model.
The comparison of ionic bond, covalent bond and metallic bond is discussed below.
| Ionic Bond | Covalent Bond | Metallic Bond |
| The transfer of electrons between two atoms having different electro negativities forms this bond. | This bond is formed by the mutual sharing of electrons between same or different elements. | This bond is formed due to the attraction between kernels and the mobile electrons in a metal lattice. |
| This is a strong bond due to electrostatic force of attraction. | This is also a fairly strong bond because the electron pair is strongly attracted by two nuclei. | This is a weak bond due to the simultaneous attraction of the electrons by a large number of kernels |
| This is a non-directional bond. | This is a directional bond. | This is a non-directional bond. |
| This bond makes substances hard and brittle. | This bond makes substances hard and incompressible. | This bond make substances malleable and ductile. |
Compounds.A pure chemical compound is a chemical substance that is composed of a particular set of molecules or ions that are chemically bonded. Two or more elements combined into one substance through a chemical reaction, such as water, form a chemical compound. All compounds are substances, but not all substances are compounds. A chemical compound can be either atoms bonded together in molecules or crystals in which atoms, molecules or ions form a crystalline lattice. Compounds made primarily of carbon and hydrogen atoms are called organic compounds, and all others are called inorganic compounds. Compounds containing bonds between carbon and a metal are called organometallic compounds.
Chemical compounds have a unique and defined structure, which consists of a fixed ratio of atoms held together in a defined spatial arrangement by chemical bonds. Chemical compounds can be:
· molecular compounds held together by covalent bonds
· salts held together by ionic bonds
· intermetallic compounds held together by metallic bonds
· complexes held together by coordinate covalent bonds.
Pure chemical elements are not considered chemical compounds, even if they consist of diatomic or polyatomic molecules (molecules that contain only multiple atoms of a single element, such as H2 or S8).
Chemical substances are often called 'pure' to set them apart from mixtures. A common example of a chemical substance is pure water; it always has the same properties and the same ratio of hydrogen to oxygen whether it is isolated from a river or made in a laboratory. Other chemical substances commonly encountered in pure form are diamond (carbon), gold, table salt (sodium chloride), and refined sugar (sucrose). Simple or seemingly pure substances found in nature can in fact be mixtures of chemical substances. For example, tap water may contain small amounts of dissolved sodium chloride and compounds containing iron, calcium, and many other chemical substances. Pure distilled water is a substance, but seawater, since it contains ions and complex molecules, is a mixture.
Chemical Mixtures.A mixture is a material system made up of two or more different substances, which are mixed but not combined chemically. A mixture refers to the physical combination of two or more substances in which the identities of the individual substances are retained. Mixtures take the form of alloys, solutions, suspensions, and colloids.
Heterogeneous Mixtures: a heterogeneous mixture is a mixture of two or more chemical substances (elements or compounds), where the different components can be visually distinguished and easily separated by physical means. Examples include:
· mixtures of sand and water
· mixtures of sand and iron filings
· a conglomerate rock
· water and oil
· a salad
· trail mix
· mixtures of gold powder and silver powder
Homogenous Mixtures: a homogeneous mixture is a mixture of two or more chemical substances (elements or compounds), where the different components cannot be visually distinguished. Often separating the components of a homogeneous mixture is more challenging than separating the components of a heterogeneous mixture.
Here are some homogeneous mixtures:
· Water itself is an example of a homogeneous mixture. It often contains dissolved minerals and gases, but these are dissolved throughout the water. Tap water and rain water are both homogeneous, even though they may have different levels of dissolved minerals and gases.
· The air that you breathe is a homogeneous mixture of oxygen, nitrogen, argon, and carbon dioxide, along with other elements in smaller amounts. Because each layer of the Earth’s atmosphere has a different density, each layer of air is its own homogeneous mixture.
· An alloy is a metal comprised of two pure metals. Alloys such as steel and bronze are homogeneous mixtures of two metals.
· Chemical solutions are usually homogeneous mixtures. The exception would be solutions that contain another phase of matter. For example, you can make a homogeneous solution of sugar and water, but if there are crystals in the solution, it becomes a heterogeneous mixture.
All properties of matter are either physical or chemical properties and physical properties are either intensive or extensive.
Extensive properties, such as mass and volume, depend on the amount of matter being measured.
Intensive properties, such as density and color, do not depend on the amount of the substance present.
Both extensive and intensive properties are physical properties, which means they can be measured without changing the substance's chemical identity. For example, the freezing point of a substance is a physical property: when water freezes, it's still water (H2O) – it's just in a different physical state.
Physical properties are properties that can be measured or observed without changing the chemical nature of the substance. Some examples of physical properties are:
· color (intensive)
· density (intensive)
· volume (extensive)
· mass (extensive)
· boiling point (intensive): the temperature at which a substance boils
· melting point (intensive): the temperature at which a substance melts
Chemical properties can be measured only by changing a substance's chemical identity.
Here are several examples of chemical properties:
· Heat of combustion is the energy released when a compound undergoes complete combustion (burning) with oxygen. The symbol for the heat of combustion is ΔHc.
· Chemical stability refers to whether a compound will react with water or air (chemically stable substances will not react). Hydrolysis and oxidation are two such reactions and are both chemical changes.
· Flammability refers to whether a compound will burn when exposed to flame. Again, burning is a chemical reaction – commonly a high-temperature reaction in the presence of oxygen.
· The preferred oxidation state is the lowest-energy oxidation state that a metal will undergo reactions in order to achieve (if another element is present to accept or donate electrons).
There are two types of change in matter: physical change and chemical change.
Physical change is a process that does not cause a substance to become a fundamentally different substance.
Chemical change is a process that causes a substance to change into a new substance with a new chemical formula. Chemical changes are also known as chemical reactions. The "ingredients" of a reaction are called reactants, and the end results are called products.
Chemical reaction is a process involving the breaking or making of interatomic bonds and the transformation of a substance (or substances) into another. There are five different types of chemical reactions.
1. Combination reaction is a reaction involving the formation of compound from two or more substances is called a combination reaction. For example, formation of sodium chloride.
2Na(s) + Cl2(g) → 2NaCl(s)
2. Combustion reaction is a process of burning and most combustion involves reaction with oxygen. For example, the combustion of liquid ethanol called ethyl alcohol is given below:
C2H5OH(l) + O2(g) → CO2(g) + H2O(g)
3. Decomposition reaction is a process in which one compound decomposes or splits to form two or simpler compounds or elements. For example, decomposition of calcium carbonate is given below:
CaCO3(s) → CaO(s) + CO2(g)
4. Single replacement reaction. An element reacts with a compound and results in the displacement of an element or group from the compound. For example, in this reaction, Zn substitutes for Cu.
Zn(s) + CuCl2(aq) → ZnCl2(aq) + Cu(s)
5. Double replacement reaction is also called metathesis reaction, which involves the exchange of two groups or two ions among the reactants. This reaction often results in an insoluble product from soluble reactants and the insoluble compound formed is called a precipitate. For example, reaction involves the formation of a precipitate of AgCl.
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
Signs of chemical reactions. It is possible to know, was whether a chemical reaction between reactants or not:
- Color change (for example, light iron covered in moist air brownish tinged oxide iron is a chemical reaction interaction of iron with oxygen).
- Precipitation, for example, if in a lime solution (a solution of calcium hydroxide) we can get if to pass the carbon dioxide in a solution of calcium hydroxide.
- Get a gas (for example, if drop citric acid to baking soda, it will get out carbon dioxide).
- The weak-disturbed formation of substances (reactions in which one of the products of the reaction is water).
- Glow solution and others.
Дата добавления: 2018-11-25; просмотров: 1299;
