Electrochemical Cells – What electroanalytical chemists use
Electrochemical books define two types of electrochemical cell: a galvanic (or voltaic cell) and an electrolytic cell. However for electroanalytical purposes an electrochemical cell can be more broadly defined as the combination of a minimum of two electrodes immersed in a solution containing the analyte, with an external connection between the electrodes to complete the electrical circuit. Such a basic cell is illustrated in figure (1) below.
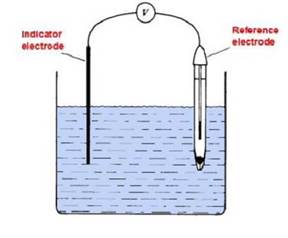
Figure 1 – Basic electrochemical cell
Galvanic (or voltaic) Cells
An electrochemical cell which spontaneously produces current when the electrodes are connected. These types of cells are important in potentiometry and as batteries but have limited use in analytical measurement. A typical galvanic cell is the Daniell cell shown in figure (2) below.
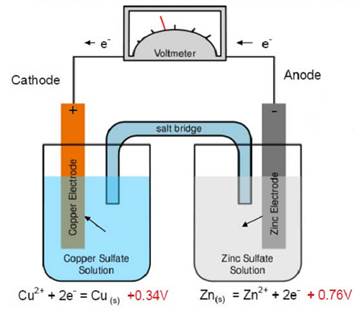
Figure 2 - Daniell cell
When a zinc half cell (Zn metal in contact with a solution of Zn2+) is connected electrically to a copper half cell, there is a spontaneous reaction whereby the Zn metal electrode dissolves with an equivalent quantity of Cu+ being deposited onto the Cu metal electrode. The reaction continues until either all of the Zn has dissolved or all of the Cu+ have been deposited.
Electrolytic Cells
There are electrochemical cells where a chemical reaction is brought about by applying a voltage from an external power supply in excess to that generated by any natural Galvanic mechanism. The resultant current flow can be measured and used for analytical measurement. These types of cells are important in voltametry, amperometry and coulometry. A typical cell is illustrated in figure (3) below.
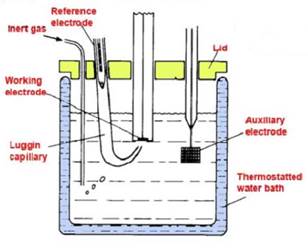
Figure 3 – Typical electrolytic cell
Figure shows the cell arrangement for a typical potentiostatic arrangement. The current generated by the electrochemical reaction carried out is passed between the working and the auxiliary electrode, whilst the reference electrode is placed close to the working electrode so that the potential at the working electrode can be maintained at a set value.
Electrodes
In both types of these cells the electrode at which oxidation occurs is the anode and that at which reduction occurs is the cathode.
In the galvanic cell the cathode reaction is given by
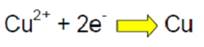 (equation 1)
(equation 1)
and the another reaction by
 (equation 2)
(equation 2)
The solutions are contained in separate beakers and connected by a salt bridge (a salt bridge allows charge transfer but prevents mixing of the solutions). If we place a zinc electrode into the zinc solution and a copper electrode in the copper solution and connect the two together we have a voltaic cell. If an ammeter is connected between the two electrodes (in series) indicates a flow of current from the reduction of copper at the cathode. The released current flows through the wire and oxidises the zinc at the anode. These reactions are referred to as half cell reactions.
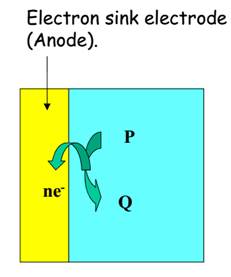
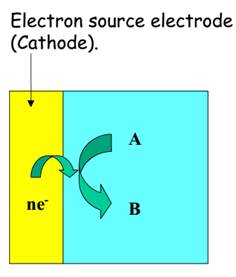
Oxidation or de-electronation. Reduction or electronation.
P = reductant (electron donor) A = oxidant (electron acceptor)
Q = Product B = Product
Figure 4 -
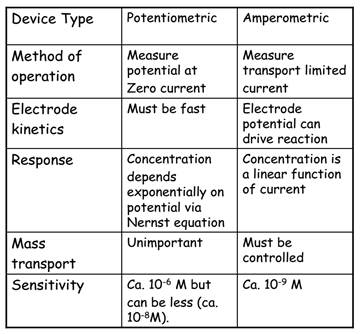
In potentiometry an interfacial ET reaction is in equilibrium and the interfacial potential is governed by the Nernst equation.
In voltammetry an analyte species is oxidised or reduced at an indicator electrode giving rise to a current flow which is directly proportional to the bulk analyte concentration.
Дата добавления: 2018-11-25; просмотров: 737;
