The Combined Gas Law
The combined gas law is a gas law which combines Charles's law, Boyle's law, and Gay-Lussac's law. These laws each relate one thermodynamic variable to another mathematically while holding everything else constant. Charles' law states that volume and temperature are directly proportional to each other as long as pressure is held constant. Boyle's law asserts that pressure and volume are inversely proportional to each other at fixed temperature. Finally, Gay-Lussac's law introduces a direct proportionality between temperature and pressure as long as it is at a constant volume. The inter-dependence of these variables is shown in the combined gas law, which clearly states that: The ratio between the pressure-volume product and the temperature of a system remains constant.
This can be stated mathematically as 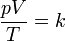
where:p is the pressure; V is the volume (L); T is the temperature measured in kelvins; k is a constant (with units of energy divided by temperature).
For comparing the same substance under two different sets of conditions, the law can be written as:
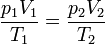
Ideal Gas Law
The ideal gas law is the combination of the three simple gas laws. By setting all three laws directly or inversely proportional to Volume, you get: V ∝ nTP
Next replacing the directly proportional to sign with a constant(R) you get: V = RnTP
And finally get the equation: PV = nRT
where P= the absolute pressure of ideal gas
V= the volume of ideal gas
n = the amount of gas (moles)
T = the absolute temperature
R = the gas constant= 8.3145 Joules · mol-1 · K-1 (SI Unit) = 0.082057 L · atm·K-1 · mol-1
All of these gas laws are based on “ideal” gases. Ideal gases have the following properties:
1. All gas molecules are in motion, and move randomly.
2. Each time the gas particles collide, kinetic energy is conserved (this is called elasticity).
3. The volume of the molecules of gas is negligible (meaning so small it’s not worth stating).
4. Gases do not attract or repel other gas molecules (there are no IMAFs).
5. The kinetic energy of a gas is directly proportional to its temperature (in Kelvins), and is the same for all gases at the same temperature.
 Avogadro's Law
Avogadro's Law
Avogadro's theory; Avogadro's hypothesis is a principle stated in 1811 by the Italian chemist Amedeo Avogadro (1776-1856) that "equal volumes of gases at the same temperature and pressure contain the same number of molecules regardless of their chemical nature and physical properties". This number (Avogadro's number) is 6.022 * 1023. It is the number of molecules of any gas present in a volume of 22.41 L and is the same for the lightest gas (hydrogen) as for a heavy gas such as carbon dioxide or bromine.
The law can be stated mathematically: 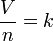 .
.
where: V is the volume of the gas.
n is the amount of substance of the gas.
k is a proportionality constant.
The most important consequence of Avogadro's law is that the ideal gas constant has the same value for all gases. This means that the constant
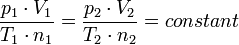
where: p is the pressure of the gas
T is the temperature of the gas has the same value for all gases, independent of the size or mass of the gas molecules.
One mole of an ideal gas occupies 22.4 liters (dm³) at STP, and occupies 24.45 litres at SATP (Standard Ambient Temperature and Pressure = 273K and 1 atm or 101.325 kPa). This volume is often referred to as the molar volume of an ideal gas. Real gases may deviate from this value. Or to put it another way "the principle that equal volumes of all gases at the same temperature and pressure contain the same number of molecules. Thus, the molar volume of all ideal gases at 0° C and a pressure of 1 atm. is 22.4 liters"
Avogadro's number is one of the fundamental constants of chemistry. It permits calculation of the amount of pure substance (mole), the basis of stoichiometric relationships. It also makes possible determination of how much heavier a simple molecule of one gas is than that of another, as a result the relative molecular weights of gases can be ascertained by comparing the weights of equal volumes.
Avogadro's number (conventionally represented by N' in chemical calculations) is now considered to be the number of atoms present in 12 grams of the carbon-12 isotope (one mole of carbon 12) and can be applied to any type of chemical entity.

Дата добавления: 2018-11-25; просмотров: 544;
