Boyle's Law: The Pressure-Volume Law
Boyle's law or the pressure-volume law states that the volume of a given amount of gas held at constant temperature varies inversely with the applied pressure when the temperature and mass are constant.
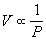
Another way to describing it is saying that their products are constant.
PV = C
When pressure goes up, volume goes down. When volume goes up, pressure goes down.
From the equation above, this can be derived: P1V1 = P2V2 = P3V3
This equation states that the product of the initial volume and pressure is equal to the product of the volume and pressure after a change in one of them under constant temperature.
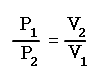
 Charles' Law: The Temperature-Volume Law
Charles' Law: The Temperature-Volume Law
This law states that the volume of a given amount of gas held at constant pressure is directly proportional to the Kelvin temperature.
V  T
T
Same as before, a constant can be put in: V / T = C
As the volume goes up, the temperature also goes up, and vice-versa.
Also same as before, initial and final volumes and temperatures under constant pressure can be calculated.
V1 / T1 = V2 / T2 = V3 / T3
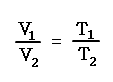
 Gay-Lussac's Law: The Pressure Temperature Law
Gay-Lussac's Law: The Pressure Temperature Law
This law states that the pressure of a given amount of gas held at constant volume is directly proportional to the Kelvin temperature.
P 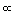 T
T
Same as before, a constant can be put in: P / T = C
As the pressure goes up, the temperature also goes up, and vice-versa. Also same as before, initial and final volumes and temperatures under constant pressure can be calculated.
P1 / T1 = P2 / T2 = P3 / T3
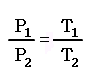
Дата добавления: 2018-11-25; просмотров: 793;
