Chemical Reactions of Electrolytes
Evidence for the existence of ions in aqueous solutions of electrolytes is furnished by well known reactions in inorganic chemistry.
When solutions of electrolytes are combined, the cations and anions will meet each other. When the ions are indifferent of each other, there is no reaction. However, some cations and anions may form a molecule or solid, and thus the cations and anions change partners. These are called Metathesis reactons, which include:
· Solid formation (or precipitation) reactions: the cations and anions form a less soluble solid, resulting in the appearance of a precipitate. Most precipitations take place when the anions and cations of two ionic compounds change partners. For example, an aqueous solution of lead(II) nitrate reacts with an aqueous solution of potassium iodide to yield an aqueous solution of potassium nitrate plus an insoluble yellow precipitate of lead iodide:
Pb(NO3)2 (aq) + 2 KCl (aq)  PbCl2 (s) + 2 KNO3 (aq)
PbCl2 (s) + 2 KNO3 (aq)
· Neutralization reactions: H+ of an acid and OH- of a base combine to give the neutral water molecule and salt:
NaOH(aq) + HCl(aq)  NaCl(aq) + H2O(aq)
NaCl(aq) + H2O(aq)
When one gram equivalent of a strong acid is neutralized by one gram equivalent of a strong base, the heat evolved is always the same, i.e., 13.7 kcal. This can be explained on the basis of Arrhenius theory that an acid furnished H+ ions and base OH- ions when dissolved in water and the process of neutralization involves the common reaction:
H+ + OH-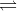 H2O + 13.7 kcal.
H2O + 13.7 kcal.
Thus, heat of neutralization is actually the heat of formation of H2O from H+ and OH- ions.
· Gas formation reactions: When neutral gaseous molecules are formed in a reaction, they leave the solution forming a gas:
K2S (aq) + 2HCl (aq)  2KCl (aq) + H2S ↑
2KCl (aq) + H2S ↑
References:
1. The abstract of the lecture.
2. intranet.tdmu.edu.ua/auth.php
3. Atkins P. W. Physical chemistry / P.W. Atkins. – New York, 1994. – P.299‑307.
4. Cotton F. A. Chemical Applications of Group Theory / F. A. Cotton. ‑ John Wiley & Sons : New York, 1990.
5. Girolami G. S. Synthesis and Technique in Inorganic Chemistry / G. S. Girolami, T. B. Rauchfuss, R. J. Angelici. ‑ University Science Books : Mill Valley, CA, 1999.
6. Russell J. B. General chemistry / J B. Russell. New York.1992. – P. 550‑599.
7. Lawrence D. D. Analytical chemistry / D. D. Lawrence. –New York, 1992. – P. 218–224.
Дата добавления: 2018-11-25; просмотров: 452;
