Oxidation-Reduction Reactions
Redox reactions involve complementary processes of oxidation and reduction, and can be identified on the basis of one or more of four definitions of oxidation and reduction.
The most commonly used definition refers to electron transfer. (OIL RIG)
Other definitions relate to oxygen transfer, hydrogen transfer and changes in oxidation numbers.
1) Oxygen Transfer:
· Reduction is the loss of oxygen
· Oxidation is the gain of oxygen

2) Hydrogen Transfer:
· Reduction is the gain of hydrogen
· Oxidation is the loss of hydrogen
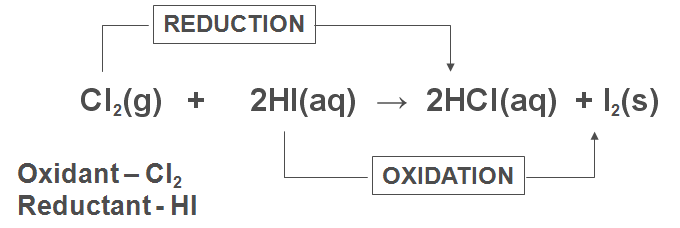
3) Electron Transfer:
· Oxidation is loss of electrons
· Reduction is gain of electrons
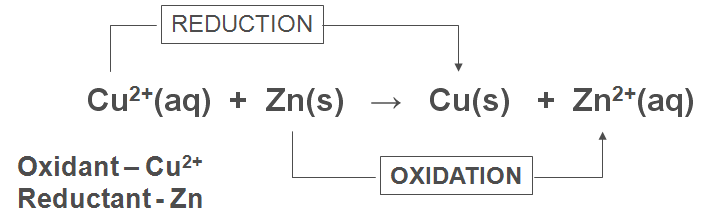
4) Changes In Oxidation Numbers:
A key stage in the production of sulphuric acid is the conversion of SO2 to SO3 according to the equation:
2SO2(g) + O2(g) → 2SO3(g)
This is a redox reaction but is not readily identified as such by the previous definitions of oxidation and reduction. To overcome this difficulty numbers called oxidation numbers can be used.
Oxidation-Reduction Reactionsare all reactions that involve the change of an oxidation number, and transfer of electrons among the reacting substances.
Oxidation is the loss of electrons. When a species loses electrons it is said to be oxidized:
Fe3+ – 1e àFe2+
Reduction is the gain of electrons. When a species gains electrons it is said to be reduced:
MnO4- + 8H+ + 5e à Mn2+ + 4H2O
Overall redox equations can be created by combining the half-equations for the oxidation process and reduction processes, after multiplying all the coefficients of the species in one of the half-equations by a factor which ensures that the number of electrons gained is equal to the number of electrons lost.
1) Balance all elements except hydrogen and oxygen in the half equation
Fe3+ à Fe2+
2) Balance the oxygen atoms by adding water:
MnO4- à Mn2+ + 4H2O
3) Balance the hydrogen atoms by adding H+ ions
MnO4- + 8H+ à Mn2+ + 4H2O
4) Now balance the charges
Fe3+ – 1e à Fe2+ 1 5 oxidation
MnO4- + 8H+ + 5e à Mn2+ + 4H2O 5 1 reduction
5) Multiplying all coefficients in the oxidation reaction by 5:
5Fe3+ – 5e à 5Fe2+
means that 5 electrons are gained and five are lost
and overall equation:
MnO4- + 8H+ + 5Fe2+ à Mn2+ + 4H2O + 5Fe3+
Problem 3. Determine which element is oxidized and which element is reduced in the following reactions (be sure to include the OS of each):
Zn + 2H+ → Zn2+ + H2
2Al + 3Cu2+→2Al3+ +3Cu
CO32- + 2H+→ CO2 + H2O
Solution:
Zn is oxidized (Oxidation number: 0 → +2); H+ is reduced (Oxidation number: +1 → 0)
Al is oxidized (Oxidation number: 0 → +3); Cu2+ is reduced (+2 → 0)
This is not a redox reaction because each element has the same oxidation number in both reactants and products: O= -2, H= +1, C= +4.
Дата добавления: 2018-11-25; просмотров: 614;
