Oxidizing and reducing agents
Oxidizing and reducing agents are key terms used in describing the reactants in redox reactions that transfer electrons between reactants to form products. Oxidizing and reducing agents are important in industrial applications. They are used in processes such as purifying water, bleaching fabrics, and storing energy (such as in batteries and gasoline). Oxidizing and reducing agents are especially crucial in biological processes such as metabolism and photosynthesis. For example, organisms use electron acceptors such as NAD+ to harvest energy from redox reactions as in the hydrolysis of glucose:
C6H12O6 + 2ADP + 2P + 2NAD+ → 2CH3COCO2H + 2ATP + 2NADH
All combustion reactions are also examples of redox reactions. A combustion reaction occurs when a substance reacts with oxygen to create heat. One example is the combustion of octane, the principle component of gasoline:
2C8H18 (l)+25O2 (g) → 16CO2 (g) + 18H2O (g)
Combustion reactions are a major source of energy for modern industry.
A species which can accept (+ne) electrons from another species is an oxidising agent(Cl2, KMnO4, K2Cr2O7, HNO3 concentrated, H2O2). Oxidising agents are reduced during redox reactions. MnO4- is the oxidizing agent in the above reaction.
Atoms, ions, and molecules that have an unusually large affinity for electrons tend to be good oxidizing agents. An oxidizing agent earns its title by its ability to take electrons from another substance. A strong oxidizing agent has a strong attraction for electrons. Conversely a weak oxidizing agent attracts electrons only slightly. Elemental fluorine, for example, is the strongest common oxidizing agent. F2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. Other good oxidizing agents include O2, O3, and Cl2, which are the elemental forms of the second and third most electronegative elements, respectively.
Another place to look for good oxidizing agents is among compounds with unusually large oxidation states, such as the permanganate (MnO4-), chromate (CrO42-), and dichromate (Cr2O72-) ions, as well as nitric acid (HNO3), perchloric acid (HClO4), and sulfuric acid (H2SO4). These compounds are strong oxidizing agents because elements become more electronegative as the oxidation states of their atoms increase.
A species which can donate (–ne) electrons to another species is a reducing agent(metals, SO2, H2S gas, Na2SO3, SnCl2 solution). Reducing agents are oxidised during redox reactions. Fe2+ is the reducing agent in the above reaction.
Good reducing agents include the active metals, such as sodium, magnesium, aluminum, and zinc, which have relatively small ionization energies and low electro-negativities. Metal hydrides, such as NaH, CaH2, and LiAlH4, which formally contain the H- ion, are also good reducing agents.
Some compounds can act as either oxidizing agents or reducing agents. One example is hydrogen gas, which acts as an oxidizing agent when it combines with metals and as a reducing agent when it reacts with nonmetals.
2 Na(s) + H2(g)  2 NaH(s) 2 NaH(s)
|
H2(g) + Cl2(g)  2 HCl(g) 2 HCl(g)
|
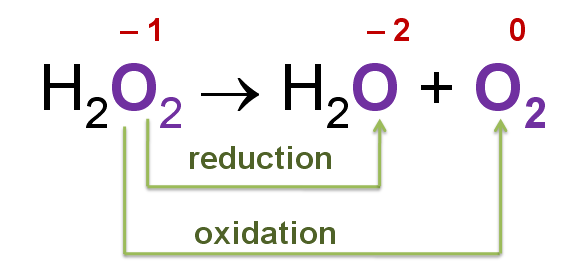
Another example is hydrogen peroxide, in which the oxygen atom is in the -1 oxidation state. Because this oxidation state lies between the extremes of the more common 0 and -2 oxidation states of oxygen, H2O2 can act as either an oxidizing agent or a reducing agent.

The main property of oxidising agent and reducing agent are their equivalent weight (mass) that react with one mole of electrons in a redox reaction:
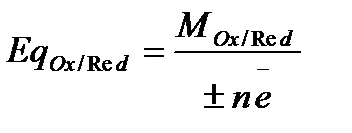
To predict the products of a redox reaction, look at the reagents given to see if there is both an oxidizing agent and a reducing agent. When a problem mentions an acidic or basic solution, it is probably redox.
| Common oxidizing agents | Products formed |
| MnO4- in acidic solution MnO2 in acidic solution MnO4- in neutral or basic solution Cr2O7- in acidic solution HNO3, concentrated HNO3, dilute H2SO4, hot, concentrated metalic ions free halogens Na2O2 HC1O4 H2O2 | Mn2+ Mn2+ MnO2 (s) Cr3+ NO2 NO SO2 metalous ions halide ions NaOH Cl- H2O |
| Common reducing agents | Products formed |
| halide ions (Cl-, I-, Br-) free metals (IA and IIA groups) sulfite ions (SO32-) or SO2 nitrite ions (NO2-) free halogens, dilute basic solution free halogens, conc. basic solution metalous ions H2O2 C2O42- | free halogen metal ions sulfate ions (SO42-) nitrate ions (NO3-) hypohalite ions halate ions metalic ions O2 CO2 |
Problems
1) Identify the oxidizing agent and the reducing agent in the following redox reaction:
MnO2(s) + 4H+(aq) + 2Cl−(aq) → Mn2+ (aq) + 2H2O (l) + Cl2 (g)
2) For the reaction, 2NO2 (g) + 7H2 (g) → 2NH3 (g) + 4H2O (g) is hydrogen an oxidizing agent or a reducing agent? Explain.
3) An element that is oxidized is a(n) __________ agent and an element that is reduced is a(n) __________ agent.
4) Determine the oxidizing and reducing agent of the following chemical equation for aerobic respiration:
5) For a general redox reaction involving species AA and BB, with AA losing electrons and BB gaining electrons: Is A the oxidizing or reducing agent? Is B the oxidizing or reducing agent? Which one is reduced and which one is oxidized?
6) In a redox reaction, there must be
a) an oxidizing agent and no reducing agent
b) a reducing agent and no oxidizing agent
c) a reducing agent and an oxidizing agent
d) no reducing or oxidizing agent
7) Which of the following is a strong reducing agent? Which of the following is a strong oxidizing agent? NO−3, NO, N2H4, NH3
8) Which of the following statements regarding redox reactions is incorrect?
a) Oxidation is the process of loss of electrons by a chemical species ( atom, ion or molecule).
b) Reduction is the process of gain of electrons by an atom, ion or molecule.
c) A reducing agent is one that undergo reduction
d) A reducing agent gives electrons;
9) Which of the following rules to be followed for writing ionic equations is incorrect?
a) All soluble ionic compounds involved in a chemical changes are expressed in ionic symbols and covalent substances are written in molecular form. H2O, NH3, NO2, NO, SO2, CO, CO2, etc., are expressed in molecular form.
b) The ionic compound which is highly insoluble is expressed in molecular form.
c) The ions which are common and equal in number on both sides are written on product side.
d) Besides the atoms, the ionic charges must also be balanced on both the sides.
10) Which of the following equations is not a redox reaction?
a) Fe2+ → Fe3+ + e-
b) MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
c) Zn + 2H+ + 2Cl- → Zn2+ + 2Cl- + H2
d) MnO2 + 4H++ 4Cl- → Mn2++ 2Cl- + 2H2O +Cl2
11) In the reaction MnO2 + 4HCl → MnCl2 + 2H2O +Cl2 Which of the following species acts as reducing agent?
a) MnO2
b) HCl
c) Cl-
d) None of above
12) Oxidation state of Mg in MgO is
a) -1
b) -2
c) +2
d) +1
13) Which of the following oxidation states are not possible for O?
a) -1
b) -2
c) -1/2
d) +3/2
14) Oxidation state of O in OF2 is..
a) +2
b) -2
c) -1
d) +1
15) In the case of neutral molecules, the algebraic sum of the oxidation number of all the atoms present in the molecule is.
a) zero
b) one
c) two
d) three
16) Why is it important to keep track of where the electrons go in these reactions?
17) Which are more likely to be oxidizing agents: metals or halogens? reducing agents? Why?
References
1. Petrucci, et al. General Chemistry: Principles & Modern Applications. 9th ed. Upper Saddle River, New Jersey: Pearson/Prentice Hall, 2007.
2. Sadava, et al. Life: The Science of Biology. 8th ed. New York, NY. W.H. Freeman and Company, 2007
3. Gerhart, Karen. The Origins and Essentials of Life. Dubuque: Kendall/Hunt Publishing Company, 2009.
4. Pettrucci, Ralph H. General Chemistry: Principles and Modern Applications. 9th. Upper Saddle River: Pearson Prentice Hall, 2007.
5. Oxtoby, David W., H.P. Gillis, and Alan Campion. Principles of Modern Chemistry. 6th. Belmont: Thomson Brooks/Cole, 2008.
Дата добавления: 2018-11-25; просмотров: 3925;
