What is electronegativity and oxidation number of atom?
REDOX REACTIONS
A chemical change or chemical reaction is a process in which one or more pure substances are converted into one or more different pure substances. Classically, chemical reactions encompass changes that strictly involve the motion of electrons in forming and breaking of the chemical bonds. More often than not, redox reactions can be the culprit or the cure for many of the chemical reactions you observe in everyday life. For examples,
· The reaction taking place in batteries are redox reactions. Redox reactions take place in the batteries such that electrons transferred can pass through some external circuit so that they produce electric current
· Digestion and metabolism of food which takes place in our body in order to supply us the energy required to perform work is also takes place through a series of redox reactions.
· Ordinary bleach oxidise the substances that stain fabric, this makes them colourless and easier to remove from fabric.
· Redox reactions are common and vital to some of the basic functions of life, including photosynthesis, respiration, combustion, and corrosion or rusting.
Reactions involving oxidation and reduction processes are very important in our everyday world. They make batteries work and cause metals to corrode (or help to prevent their corrosion). They enable us to obtain heat by burning fuels in factories and in our bodies.
What is electronegativity and oxidation number of atom?
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The term "electronegativity" was introduced by Jöns Jacob Berzelius in 1811, in1932, Linus Pauling proposed an electronegativity scale. This gives a dimensionless quantity, commonly referred to as the Pauling scale, on a relative scale running from around 0.7 to 3.98 (hydrogen = 2.20).
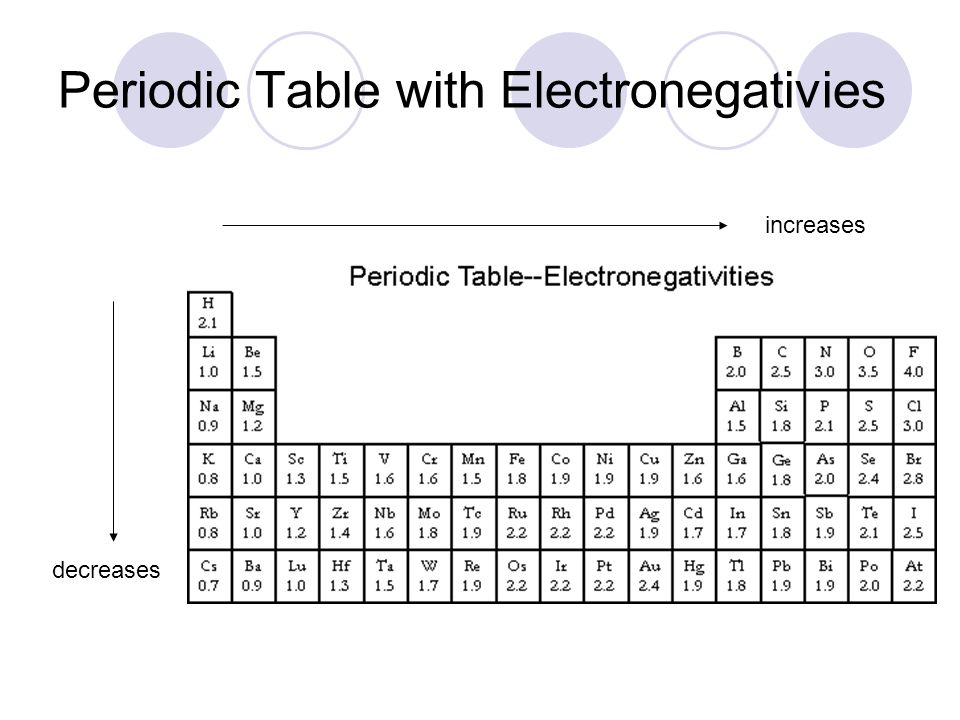
This table is the Pauling electronegativity scale. There are other ways of measuring electronegativity, such as the Mulliken scale and the Allred-Rochow scale. Linus Pauling's electronegativity scale is the most common. Note that atoms toward the upper right are more electronegative, and those to the lower left are least electronegative. Pauling did not assign electronegativities to the noble gasses because they typically do not form covalent bonds.
In general electronegativity is the measure of an atom's ability to attract electrons to itself in a covalent bond. Because fluorine is the most electronegative element, the electrons tend to "hang out" more toward the fluorine atom when fluorine is covalently bonded to other atoms. Oxygen is the 2nd most electronegative element.
When you examine a periodic table, you will find that (excluding the noble gases) the electronegativity values tend to increase as you go to the right and up. The reverse statement is that the values tend to decrease going down and to the left. This pattern will help when you are asked to put several bonds in order from most to least ionic without using the values themselves.
Electronegativity values are useful in determining if a bond is to be classified as nonpolar covalent, polar covalent or ionic. For example,
· No electronegativity difference between two atoms leads to a pure non-polar covalent bond.
· A small electronegativity difference leads to a polar covalent bond.
· A large electronegativity difference leads to an ionic bond.
What you should do is look only at the two atoms in a given bond. Calculate the difference between their electronegativity values. Only the absolute difference is important.
I. Nonpolar Covalent: This type of bond occurs when there is equal sharing (between the two atoms) of the electrons in the bond. Molecules such as Cl2, H2 and F2 are the usual examples.
Textbooks typically use a maximum difference of 0.2 – 0.5 to indicate nonpolar covalent. Since textbooks vary, make sure to check with your teacher for the value he/she wants. The ChemTeam will use 0.5.
One interesting example molecule is CS2. This molecule has nonpolar bonds. Sometimes a teacher will only use diatomics as examples in lecture and then spring CS2 as a test question. Since the electronegativities of C and S are both 2.5, you have a nonpolar bond.
II. Polar Covalent: This type of bond occurs when there is unequal sharing (between the two atoms) of the electrons in the bond. Molecules such as NH3 and H2O are the usual examples.
The typical rule is that bonds with an electronegativity difference less than 1.6 are considered polar. (Some textbooks or web sites use 1.7.) Obviously there is a wide range in bond polarity, with the difference in a C-Cl bond being 0.5 -- considered just barely polar to the difference the H-O bonds in water being 1.4 and in H-F the difference is 1.9. This last example is about as polar as a bond can get.
III. Ionic: This type of bond occurs when there is complete transfer (between the two atoms) of the electrons in the bond. Substances such as NaCl and MgCl2 are the usual examples.
The rule is that when the electronegativity difference is greater than 2.0, the bond is considered ionic.
So, let's review the rules:
1) If the electronegativity difference (usually called ∆EN) is less than 0.5, then the bond is nonpolar covalent.
2) If the ∆EN is between 0.5 and 1.6, the bond is considered polar covalent
3) If the ∆EN is greater than 2.0, then the bond is ionic.
That, of course, leaves us with a problem. What about the gap between 1.6 and 2.0? So, rule #4 is:
4) If the ∆EN is between 1.6 and 2.0 and if a metal is involved, then the bond is considered ionic. If only nonmetals are involved, the bond is considered polar covalent.
Here is an example: Sodium bromide (formula = NaBr; ENNa = 0.9, ENBr = 2.8) has a ∆EN = 1.9. Hydrogen fluoride (formula = HF; ENH = 2.1, ENF = 4.0) has the same ∆EN. We use rule #4 to decide that NaBr has ionic bonds and that HF has a polar covalent bond in each HF molecule.
In binary compounds the element with the largest electronegativity is assigned a negative charge and the element with the smallest electronegativity is assigned a positive charge. In order to keep track of the electrons involved, we use a special counter, called the oxidation number.

The oxidation number is the total number of electrons (electron) that an atom either gains or loses in order to form a chemical bond (chemical bonding) with another atom.
Each atom that participates in an oxidation-reduction reaction (q.v.) is assigned an oxidation number that reflects its ability to acquire, donate, or share electrons. The iron ion Fe3+, for example, has an oxidation number of +3 because it can acquire three electrons to form a chemical bond, while the oxygen ion O2− has an oxidation number of −2 because it can donate two electrons. In an electronically neutral substance, the sum of the oxidation numbers is zero; for example, in hematite (Fe2O3) the oxidation number of the two iron atoms (+6 in total) balances the oxidation number of the three oxygen atoms (−6).
Certain elements assume the same oxidation number in different compounds; fluorine, for example, has the oxidation number −1 in all its compounds. Others, notably the nonmetals and the transition elements, can assume a variety of oxidation numbers; for example, nitrogen can have any oxidation number between −3 (as in ammonia, NH3) and +5 (as in nitric acid, HNO3).
In the nomenclature of inorganic chemistry, the oxidation number of an element that may exist in more than one oxidation state is indicated by a roman numeral in parentheses after the name of the element e.g., iron (II) chloride (FeCl2) and iron (III) chloride (FeCl3).
In determining the OS of an atom, there are seven guidelines to follow:
1) The oxidation number of an atom is zero in a neutral substance that contains atoms of only one element. Thus, the atoms in O2, O3, P4, S8, and aluminum metal all have an oxidation number of 0.
2) The oxidation number of monatomic ions is equal to the charge on the ion. The oxidation number of sodium in the Na+ ion is +1, for example, and the oxidation number of chlorine in the Cl- ion is -1.
3) The oxidation number of hydrogen is +1 when it is combined with a nonmetal. Hydrogen is therefore in the +1 oxidation state in CH4, NH3, H2O, and HCl.
4) The oxidation number of hydrogen is -1 when it is combined with a metal. Hydrogen is therefore in the -1 oxidation state in LiH, NaH, CaH2, and LiAlH4.
5) The metals in Group IA form compounds (such as Li3N and Na2S) in which the metal atom is in the +1 oxidation state.
6) The elements in Group IIA form compounds (such as Mg3N2 and CaCO3) in which the metal atom is in the +2 oxidation state.
7) Oxygen usually has an oxidation number of -2. Exceptions include molecules and polyatomic ions that contain O-O bonds, such as O2, O3, H2O2, and the O22- ion.
8) The nonmetals in Group VIIA often form compounds (such as AlF3, HCl, and ZnBr2) in which the nonmetal is in the -1 oxidation state.
9) The sum of the oxidation numbers of the atoms in a molecule is equal to the charge on the molecule.
10) The most electronegative element in a compound has a negative oxidation number.
Problem 1. What is the oxidation state of uranium, U, in the uranyl cation, UO22+?
If U has an oxidation state of X, then we have
X + 2 * (-2)(each oxygen atom contributes -2) = +2. This gives X = 6.
Uranium can exist in all the listed oxidation states.
Problem 2. What is the oxidation state of the sulphur atom in sulphuric acid, H2SO4?
If S has an oxidation state of X, then we have
X + 4 * (-2) (each oxygen atom contributes -2) + 2 * (1) (each hydrogen atom contributes +1) = 0. The answer is therefore +6.
Дата добавления: 2018-11-25; просмотров: 876;
