AQUEOUS SOLUTIONS OF ELECTROLYTES
One of the most important properties of water is its ability to dissolve a wide variety of substances. Solutions in which water is the dissolving medium are called aqueous solutions. For electrolyte, water is the most important solvent. Ethanol, ammonia, and acetic acid are some of the non-aqueous solvents that are able to dissolve electrolytes.
One of the major discoveries of the mid-nineteenth century was Michael Faraday's demonstration that aqueous solutions of salts and some acids would conduct an electric current. In this unit we take a close look at the nature and behavior of ions in aqueous solution, with special emphasis on the electrical conductance of such solutions.
Substances that give ions when dissolved in water are called electrolytes. They can be divided into acids, bases, and salts, because they all give ions when dissolved in water. These solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively.
Some compounds when dissolved in water dissolve completely. These compounds are considered to be strong electrolytes. They are strong because they dissolve 100% of the time. Some examples are HCl, or NaOH.
Other compounds do not dissolve very well in water. Vinegar or acetic acid dissolves in water. But it does not dissociate completely. Actually vinegar only dissociates roughly 1.8%. Therefore only 1.8% of the total amount of vinegar put into the water actually breaks down into ionic form.
 Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. For example, NaCl, HNO3, HClO3, CaCl2 etc are strong electrolytes. An ionization can be represented by,
Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution. For example, NaCl, HNO3, HClO3, CaCl2 etc are strong electrolytes. An ionization can be represented by,
NaCl(s) + nH2O = Na+(aq) + Cl-(aq)
Since NaCl is an ionic solid (s), which consists of cations Na+ and anions Cl-. No molecules of NaCl are present in NaCl solid or NaCl solution. The ionization is said to be complete. The solute is one hundred percent (100%) ionized. Some other ionic solids are CaCl2, NH4Cl, KBr, CuSO4, NaCH3COO (sodium acetate), CaCO3, NaHCO3 (baking soda).
Three classes of strong electrolytes are:
· Soluble Salts are any salt that readily dissolves in water produces a strong electrolyte when it is so dissolved. A good example is sodium chloride (NaCl).
· Strong Acids: According to Arrhenius, an acid is a substance that ionizes in aqueous solution to generate H+ ions (hydrogen ions or protons). If the ionization is complete or nearly complete, the acid solution is a good conductor of electricity, and the acid is regarded as a strong acid. Some examples of strong acids in aqueous solution are:
| H2O | ||
| HCl (g) | ——> | H+(aq) + Cl-(aq) |
| H2O | ||
| HNO3 (l) | ——> | H+(aq) + NO3-(aq) |
| H2O | ||
| H2SO4 (l) | ——> | H+(aq) + HSO4-(aq) |
Sulfuric acid (H2SO4) deserves a closer look. When sulfuric acid dissolves in water, the first hydrogen dissociates completely to form protons in aqueous solution, but the second hydrogen remains bonded to the sulfate. Thus aqueous sulfuric acid contains mostly protons (H+(aq)) and hydrogen sulfate (HSO4-(aq)) ions.
· Strong Bases: According to Arrhenius, a base is a substance that ionizes in aqueous solution to produce hydroxide ions (OH-). Bases that ionize completely are regarded as strong bases. The two most common strong bases are sodium hydroxide and potassium hydroxide:
| H2O | ||
| NaOH (s) | ——> | Na+(aq) + OH-(aq) |
| H2O | ||
| KOH (s) | ——> | K+(aq) + OH-(aq) |
Weak Electrolytes: Many substances will form ions in aqueous solution, but the extent of ionization is slight. For example, acetic acid (the essence of vinegar), will readily dissolve in water, but only around 1% of acetic acid molecules will ionize to form hydrogen ions and acetate ions. The remaining acetic acid molecules will remain as electrically neutral acetic acid molecules, even though they are totally dissolved in the solution. The result is a solution that is electrically conductive, but much less so than a comparative solution containing a strong electrolyte. The most common weak electrolytes are:
· Weak Acids: A weak acid is an acid that is only partially ionized in aqueous solution. Thus an aqueous solution of acetic acid (CH3COOH) contains some hydrogen ions (H+(aq)) and some acetate ions (CH3COO-(aq)), but most of the solute particles are undissociated acetic acid molecules (CH3COOH (aq)).
| H2O | ||
| CH3COOH | ——> <—— | H+(aq) + CH3COO-(aq) |
· Weak Bases: Ammonia (NH3) is the most common of the weak bases. It is a base because its aqueous solutions contain hydroxide ions (OH-(aq)). It is a weak electrolyte because only a small fraction of ammonia molecules form ions. Most of the ammonia remains as neutral ammonia molecules.
| H2O | ||
| NH3 (aq) + H2O (l) | ——> <—— | NH4+(aq) + OH-(aq) |
In a solution, NH4OH molecules are present. The fraction (often expressed as a %) that undergoes ionization depends on the concentration of the solution.
Electrolytic solutions are those that are capable of conducting an electric current. A substance that, when added to water, renders it conductive, is known as an electrolyte. A common example of an electrolyte is ordinary salt, sodium chloride. Solid NaCl and pure water are both non-conductive, but a solution of salt in water is readily conductive. A solution of sugar in water, by contrast, is incapable of conducting a current; sugar is therefore a non-electrolyte.
These facts have been known since 1800 when it was discovered that an electric current can decompose the water in an electrolytic solution into its elements (a process known as electrolysis). By mid-century, Michael Faraday had made the first systematic study of electrolytic solutions.
Faraday recognized that in order for a sample of matter to conduct electricity, two requirements must be met:
· The matter must be composed of, or contain, electrically charged particles.
· These particles must be mobile; that is, they must be free to move under the influence of an external applied electric field.
In metallic solids, the charge carriers are electrons rather than ions; their mobility is a consequence of the quantum-mechanical uncertainty principle which promotes the escape of the electrons from the confines of their local atomic environment.
In the case of electrolytic solutions, Faraday called the charge carriers ions (after the Greek word for "wanderer"). His most important finding was that each kind of ion (which he regarded as an electrically-charged atom) carries a definite amount of charge, most commonly in the range of ±1-3 units. Since positively-charged ions are attracted to a negative electrode that is traditionally known as the cathode, these are often referred to as cations. Similarly, negatively-charged ions, being attracted to the positive electrode, or anode, are called anions. (These terms were all coined by Faraday.)
In order to explain the properties of electrolytic solutions, Arrhenius put forth, in 1884, a comprehensive theory which is known as theory of Electrolytic Dissociation or Ionic Theory.
The main points of the theory are:
1) An electrolyte, when dissolved in water, breaks up into two types of charged particles, one carrying a positive charge and the other a negative charge. These charged particles are called ions. Positively charged ions are termed cations and negatively charged as anions.
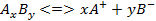
K2S <=> 2K+ + S2-
H2SO4 <=> 2H+ + SO42-
Ba(OH)2 <=> Ba2+ + 2OH-
In its modern form, the theory assumes that solid electrolytes are composed of ions which are held together by electrostatic forces of attraction. When an electrolyte is dissolved in a solvent, these forces are weakened and the electrolyte undergoes dissociation into ions. The ions are solvated.
2) The process of splitting of the molecules into ions of an electrolyte is called ionization. The fraction of the total number of molecules present in solution as ions is known as degree of ionization or degree of dissociation. It is denoted by:
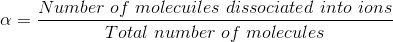
It has been observed that all electrolytes do not ionize to the same extent.
Some are almost completely ionized while others are feebly ionized. The degree of ionization depends on a number of factors.
According to the Degree of dissociation (α) electrolytes can be classified into the following:
· strong electrolytes are compounds that dissociate to a large extent ( α> 30%) into ions when dissolved in water. For example, HCl, H2SO4, HNO3, HJ, NaOH, KOH, KCl.
· medium strong electrolytes α = 2 - 30%. For istance, H2SO3, H3PO4, H3PO3.
· weak electrolytes are compounds that dissociate to only a small extent α<2%. For example, H2O, NH4OH, H2S, HCN, H2CO3.
· nonelectrolytes α = 0 are compounds that don’t dissociate into ions when dissolved in water.
The degree of ionization of an electrolyte in solution depends upon the following factors:
1) Nature of solute: When the ionisable parts of a molecule of a substance are held more by covalent bonding than by electrovalent bonding, less ions are furnished in solution. Such substances are termed weak electrolytes. H2S, HCN, NH4OH, CH3COOH are examples of this class. NaCl, Ba(NO3)2, KOH, etc., are strong electrolytes, in which the transfer of electrons seems to be more or less complete, furnish ions immediately when dissolved. Strong electrolytes are almost completely ionized in solution.
2) Nature of solvent: The main function of the solvent is to weaken the electrostatic forces of attraction between the two ions and separate them. The force of attraction holding the ions together in any medium is expressed as:
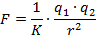
where K is the dielectric constant of medium.
Any solvent which has high value of dielectric constant has the capacity of separating ions. Water is considered to be the best solvent as it has the highest dielectric constant. The dielectric constants of some of the solvents are given below at 25°C.
| Solvent | Dielectric Constant |
| Water | |
| Methyl alcohol | |
| Ethyl alcohol | |
| Acetone |
3) Dilution: The extent of ionization of an electrolyte is inversely proportional to the concentration of its solution. Thus, degree of ionization increases with the increase of dilution of the solution, i.e., decreasing the concentration of the solution.
4) Temperature: The degree of ionization increases with the increase of temperature. This is due to the fact that at higher temperature molecular speed is increased which overcomes the forces of attraction between the ions.
3) Ions present in solution constantly re-unite to form neutral molecules and, thus, there is a state of dynamic equilibrium between the ionized the ionized and non-ionised molecules, i.e., AB  A+ + B-
A+ + B-
Applying the law of mass action to above equilibrium 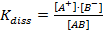
where K is known as ionization constant. The electrolytes having high value of K are termed strong electrolytes and those having low value of K as weak electrolytes.
Ostwald dilution law state:A law stating that for dilute solutions of a binary electrolyte the square of the degree of dissociation of the solute, multiplied by its concentration, and divided by one minus the degree of dissociation, is a constant for the solute.
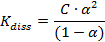
where c is the concentration of electrolyte and a is the degree of dissociation.
The Ostwald dilution law was derived by W. Ostwald in 1888. Ostwald’s experimental confirmation ofthe law was very important to the the founding of the classical theory of electrolytic dissociation.
According to Arrhenius theory of electrolyte dissociation, the molecules of an electrolyte in solution are constantly splitting up into ions and the ions are constantly reuniting to form unionized molecules. Therefore, a dynamic equilibrium exists between ions and unionized molecules of the electrolyte in solution. It was pointed out by Ostwald that like chemical equilibrium, law of mass action van be applied to such systems also. Consider a binary electrolyte AB which dissociates into A+ and B- ions and the equilibrium state is represented by the equation:
| Concentration | AB | ↔ | A+ | + | B- |
| Initially t = 0 | C | ||||
| At equilibrium | C(1-α) | C*α | C*α |
So, dissociation constant may be given as
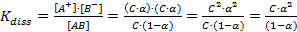 (1)
(1)
For very weak electrolytes, α << 1, thus (1 - α ) = 1
and 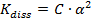 =>
=> 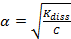 (2)
(2)
From equation (2) it is a clear that degree of ionization increases on dilution. Thus, degree of dissociation of a weak electrolyte is proportional to the square root of dilution.
Limitations of Ostwald's dilution law: The law holds good only for weak electrolytes and fails completely in the case of strong electrolytes. The value of 'α' is determined by conductivity measurements by applying the formula l/l∞. The value of 'α' determined at various dilutions of an electrolyte when substituted in Eq. (1) gives a constant value of Kdiss only in the case of weak electrolytes like CH3COOH, NH4OH, etc. the cause of failure of Ostwald's dilution law in the case of strong electrolytes is due to the following factors:
a) The law is based on the fact that only a portion of the electrolyte is dissociated into ions at ordinary dilution and completely at infinite dilution. Strong electrolytes are almost completely ionized at all dilutions and l/l∞ does not give accurate value of 'α'.
b) When concentration of the ions is very high, the presence of charges on the ions appreciably effects the equilibrium. Hence, law of mass action its simple form cannot be strictly applied in the case of string electrolytes.
4) When an electric current is passed through the electrolytic solution, the positive ions (cations) move towards cathode and the negative ions (anions) move towards anode and get discharged, i.e., electrolysis occurs. The ions are discharged always in equivalent amounts, no matter what their relative speeds are.
5) The electrolytic solutions is always neutral in nature as the total charge on one set of ions is always equal to the total charge on the other set of ions. However, it is not necessary that the number of two sets of ions must be equal always.
AB 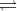 A+ + B- A+ + B-
| NaCl 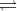 Na+ + Cl- Na+ + Cl-
| (Both ions are equal) |
AB2 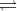 A2+ + 2B- A2+ + 2B-
| BaCl2  Ba2+ + 2Cl- Ba2+ + 2Cl-
| (Anions are double that of cations) |
A2B  2a+ + B2- 2a+ + B2-
| Na2SO4  2Na+ + 2Na+ +
| (Anions are double that of cations) |
6) The properties of electrolytes in solution are the properties of ions present in solution. For example, acidic solution always contains H+ ions while basic solution contains OH- ions and characteristic properties of solutions are those of H- ions and OH- ions respectively.
7) The ions act like molecules towards depressing the freezing point, elevating the boiling point, lowering the vapour pressure and establishing the osmotic pressure.
The abnormal behavior towards colligative properties as observed in the case of electrolytes can be explained on the basis of ionic theory. When an electrolyte is dissolved in water, the number of molecules actually dissolved due to ionization. The Van't Hoff factor,
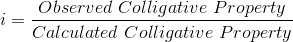
i is always more than one, i.e., i = 1 + (n-1)*α where 'n' is the number of ions produced by the ionization of one molecule of the electrolyte and 'α' is the degree of ionization.
8) The conductively of the electrolytic solution depends on the nature and number of ions as the current is carried through solution by the movement of ions.
Limitations of the Arrhenius′s theory:
· For the acidic or basic properties, the presence of water is absolutely necessary. Dry HCl shall not act as an acid. HCl is regarded as an acid only when dissolved in water and not in any other solvent.
· The concept does not explain acidic and basic character of substances in non-aqueous solvents.
· The neutralization process is limited to those reactions which can occur in aqueous solutions only, although reactions involving salt formation do occur in the absence of solvent.
· It cannot explain the acidic character of certain salts such as AlCl3 in aqueous solution.
· An artificial explanation is required to explain the basic nature of NH3 and metallic oxides and acidic nature of non-metal oxides.
Дата добавления: 2018-11-25; просмотров: 1376;
