ELECTROLYSIS OF AQUEOUSSOLUTIONS
There are 2 major differences here. The temperature is room temperature and there is water in the reaction.
A small number ofwater molecules ionize:
H2O(l) →H+(aq) +OH-(aq)
So all aqueous solutions have small concentrationof H+and OH-ions.
In electrolysis, when more than one type of cation or anion is present in a solution, only ONE cation and one anion are preferentially discharged. This is called selective discharge of ions.
How do you decide which ion is discharged? It depends on three factors:
1) The position of the metal (producing the cation) in the reactivity series.
2) The relative ease of discharge of an anion.
3) The concentration of the anion in the electrolyte.
2nd EXAMPLE:
ELECTROLYSIS OF BRINE (CONCENTRATED AQUEOUS SODIUM CHLORIDE):
The ions present in the electrolyte are H+ and OH- from water and Na+ and Cl- from sodium chloride.
Since sodium is more reactive than hydrogen, the H+ ions will be discharged at the cathode and hydrogen gas will evolve. And because the solution is concentrated, Cl- will be discharged and chlorine gas will evolve. But keep in mind that chlorine is soluble in water, it would take time for it to evolve and some oxygen can be formed too. Gases should be collected in an inverted measuring cylinder.
At the cathode: 2H+ + 2e- → H2
Hydrogen gas evolves. Observation is bubbles of colorless gas.
Test to make sure by approaching a lighted splint, if positive it will burn with a pop sound.
At the anode: 2Cl- - 2e- → Cl2
Chlorine gas evolves. Observation is bubbles of green gas. Test to make sure by approaching a damp blue litmus paper, if positive it will turn red then bleach.
Ions remaining in solution: This leaves us with two other ions, Na+ and OH-. They bond together forming sodium hydroxide which is an alkali and extracted later: Na+ + OH- → NaOH
Overall equation for the electrolysis of brine:
2NaCl + 2H2O → H2 + Cl2 + 2NaOH
3rd EXAMPLE:
THE ELECTROLYSIS OF COPPER(II) SULPHATE SOLUTIONUSING INERT ELECTRODES
A simple method of investigating the electrolysis of copper (II) sulphate solution is described. The formation of the products of electrolysing aqueous copper sulfate is fully explained with the appropriate electrode equations. Two experiments are described (a) with inert carbon electrodes and (b) using copper electrodes. The process of electroplating is also described. What are the products of the electrolysis of copper sulfate solution?
The electrolyte copper(II) sulfate, provides a high concentration of copper(II) ions Cu2+ and sulfate ions SO42– to carry the current during the electrolysis process. There are small concentrations of hydrogen ions H+ and hydroxide ions (OH–) from the self-ionisation of water itself
The products of electrolysing copper sulfate solution with inert electrodes (carbon/graphite or platinum) are copper metal and oxygen gas.
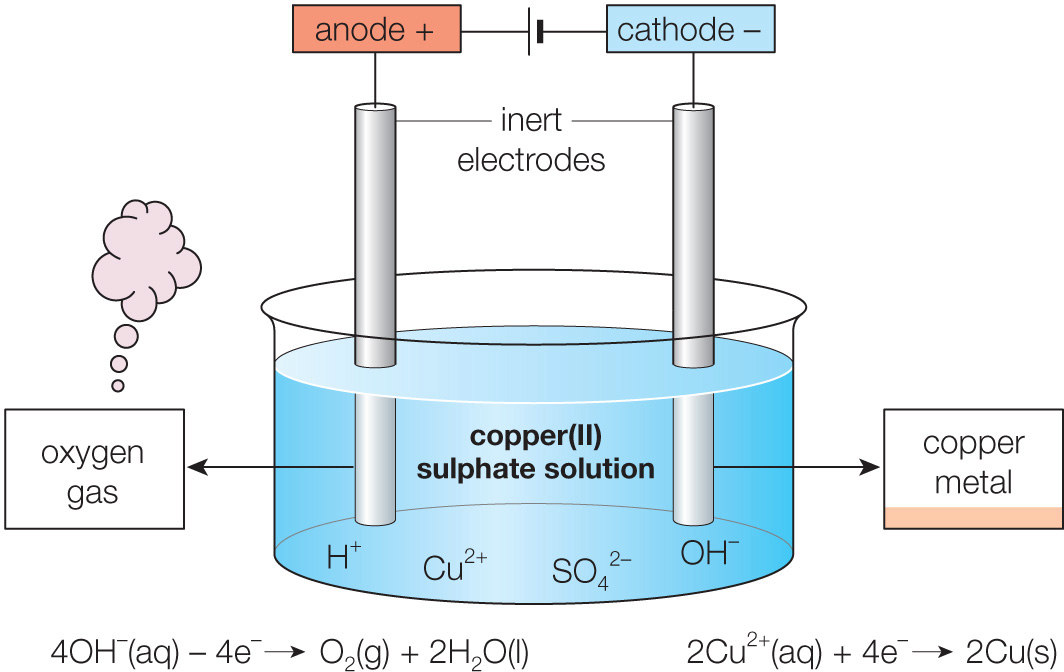
Ions present in the electrolyte: H+, OH−, Cu2+ and SO42−
CuSO4(aq)→Cu2+(aq) + SO42− (aq)
H2O(l)⇌H+(aq) + OH−(aq)
Reaction at CATHODE:
· H+ and Cu2+ ions are attracted to the cathode.
· Cu2+ ions are preferentially discharged as Cu2+ is lower in the electrochemical series than H+
· Each Cu2+ ion gains two electrons from the cathode to form one copper atom.
· Copper metal is deposited on the cathode, which resulting in the copper cathode to become larger.
Cu2+(aq) + 2e− → Cu(s)
Reaction at ANODE:
· OH− and SO42− are attracted to the anode.
· OH− ions are preferentially discharged as OH− is lower in the electrochemical series than SO42−
· And the sulfate ion is too stable and nothing happens. Instead either hydroxide ions or water molecules are discharged and oxidised to form oxygen gas.
4OH–(aq) – 4e– ==> 2H2O(l) + O2(g)
Ions remaining in solution: The ions that are removed from the solution, then, are the copper ions and the hydroxide ions. This means that the hydrogen ions and the sulphate ions remain in the solution - i.e sulphuric acid is also produced.
2H+ + SO42− ® H2SO4
Overall equation for the electrolysis of copper (II) sulfate solution:
2CuSO4 + 2H2O ® 2Cu + O2 + 2H2SO4
Дата добавления: 2018-11-25; просмотров: 2525;
