FARADAY’S LAWS ELECTROLYSIS
Michael Faraday is most famous for his work on electricity and, in 1833, he published the results of his studies of electrolysis. Faraday had made careful measurements of the amount of electricity involved during electrolysis and related it to the amount of substances produced. His work established two ‘laws’ of electrochemistry. The amount of charge carried by 1 mole of electrons is called a faraday in honour of Michael Faraday’s contribution to science.
From his experiments, Faraday deduced two fundamental laws which govern the phenomenon of electrolysis.
FARADAY’S FIRST LAW OF ELECTROLYSIS: The mass of ions liberated at an electrode is directly proportional to the quantity of electricity i.e. charge which passes through the electrolyte. Or The weight of a substance liberated from an electrolyte in a given time is 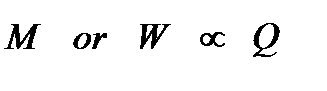 proportional to the quantity of electricity passing through the electrolyte.
proportional to the quantity of electricity passing through the electrolyte.
WorM µ Q
where W or M = amount of substance liberated in gram.
Q = quantity of electricity passed in coulomb.
Since Q = I×t
where I = Current in ampere and t = time in seconds
Hence W µI×t or W = Z×I×t = Z×Q
where Z = proportionality constant, called electrochemical equivalent.
If I = 1 ampere and t = 1 second then Z = W. Therefore electrochemical equivalent may be defined as, “The mass of substance (in grams) liberated at the electrode on passing current of 1 ampere for 1 second or on passing 1 coulomb of electricity is called electrochemical equivalent of the substance”. It's unit is gram per coulomb.
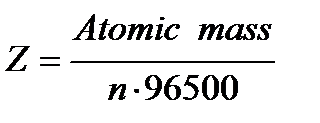 here n = number of electrons exchanged
here n = number of electrons exchanged
1F = 9500 Coulomb and E= Z × 96500
So, 1 Faraday [96500 coulomb] of electricity will produce 1 gm equivalent of Ag, Cu and Al at cathode.
Coulomb is the unit of electrical charge.
96500Coulombs = 6.023×1023 electrons = 1 mole electrons.
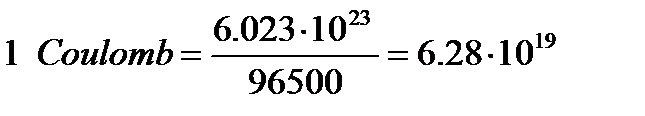 electrons,
electrons,
or 1 electronic charge = 1.6×10-19 Coulomb.
With the help of the first law of electrolysis we are able to calculate:
1. the value of electrochemical equivalents of different substances; and
2. the mass of different substances produced by passing a known quantity of electricity through their solutions.
FARADAY’S SECOND LAW OF ELECTROLYSIS:This law states that the amounts of different substances liberated by the same quantity of electricity passing through their electrolytic solution are directly proportional to their chemical equivalent masses (chemical equivalent mass of metal can be obtained by dividing its atomic mass with number of electrons required to reduce its cation). Or it can also be stated as follows: when same quantity of electricity is passed through different electrolytes connected in series then the masses of the substances liberated at the electrodes are in the ratio of their chemical equivalent masses (atomic mass + Number of electrons required to form the product) or the ratio of their electrochemical equivalents.
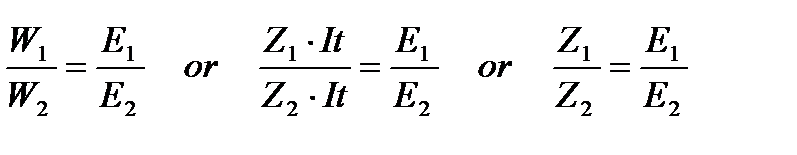
Thus the electrochemical equivalent (Z) of an element is directly proportional to its equivalent weight (E).
For example, if the two electrolytic cells A (containing AgNO3 solution) and B (containing CuSO4 solution) are connected in series and same quantity of electricity is passed through the cells. Then the ratio of the mass of copper deposited at cathode in electrolytic cell B (X g) to that of silver deposited in cell A (Y g) is equal to the ratio of their chemical equivalent masses.
Mass of Cu (x) / Mass of Ag (y) =
= Chemical Equivalent Mass of Cu / Chemical Equivalent Mass of Ag =
= Z Cu / Z Ag
Now, each copper (Cu2+) ion requires 2 electrons to form Cu and each Ag+ needs 1 electron to form Ag. Thus, chemical equivalent mass of Cu is 63.5/2 and that of Ag is 108/1.
Thus, the ratio 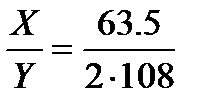
Most stoichiometric problems involving electrolysis can be solved without explicit use of Faraday's laws. The "chemistry" in these problems is usually very elementary; the major difficulties usually stem from unfamiliarity with the basic electrical units:
· current (amperes) is the rate of charge transport; 1 amp = 1 c/sec.
· power (watts)is the rate of energy production or consumption;
1 w = 1 J/sec = 1 volt-amp; 1 watt-sec = 1 J, 1 kw-h = 3600 J.
Дата добавления: 2018-11-25; просмотров: 1004;
