ELECTROLYSIS OF MOLTEN
Recall: A simple binary ionic compounds contains only two elements – a metal and a non-metal. When the ionic compound is in the molten state, the locked ions within the ionic structure will be free to move about (conduct electricity).
A typical setup for electrolysis of molten compounds is shown below:
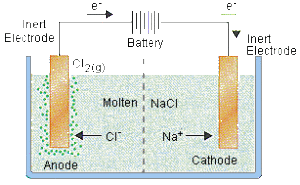
The metallic ions (cations – Me+n) will be discharged at the cathode to form a metal atom. The metallic ions are REDUCED to metal at the cathode (because they gain electrons)
Me+n+ ne− →Me°
The non-metallic ions (anions – Nn+) will be discharged at the anode to form a non-metallic atom. The non-metallic ions are OXIDISED to non-metallic atom at the anode (because they lose electrons)
Nn+ → N° + ne−
1st EXAMPLE:
THE ELECTROLYSIS OF MOLTEN LEAD(II) BROMIDE
A simple method of investigating the electrolysis of molten lead(II) bromide is described. The formation of the products of electrolysing molten lead bromide is fully explained with the appropriate electrode equations. What are the products of the electrolysis of molten lead bromide?
You can electrolyse molten compounds as long as they are ionic compounds, so that on melting there free ions to move to carry the current to facilitate the electrolysis process of splitting the compound into its constituent elements.
Molten salts with carbon electrodes:Inert carbon (graphite) electrodes are dipped into molten salt which has been strongly heated in a crucible. It is difficult to collect the gases at the electrodes! The salts may be very high melting, so sometimes a small amount of another salt impurity is added to lower the melting point.
The electrolyte molten lead(II) bromide PbBr2(l), provides a high concentration of lead(II) ions Pb2+ and bromide ions Br– to carry the current during the electrolysis process. Remember that melting an ionic compound breaks down the strong ionic bonding sufficiently to allow the ions to freely move around and carry the electric current. The electrolysis will only take place when electricity is passed through the molten lead bromide.
The electrolysis of molten salts is not as complicated as in aqueous solutions, because the electrolysis products of water are not an issue.
Although the graphite rods should be clamped as low as possible in the crucible, they should not be so low as to risk a short-circuit in the pool of liquid lead which collects at the bottom.
The solidified lead(II) bromide can be scraped out of the second crucible afterwards, so that it can be used again for this demonstration.
Lead (II) bromide is used because it melts at an unusually low temperature for an ionic compound (373 °C). Lead can be fairly safely handled afterwards (taking care to ensure that hands are washed after any contact with the metal). Bromine is a coloured acidic gas with a characteristic smell.
Electrolysis is not possible with solid lead(II) bromide. This is because the ions are held in a three-dimensional lattice, unable to move freely to the electrodes. Melting enables the ions to become mobile and to travel to the respective electrodes.
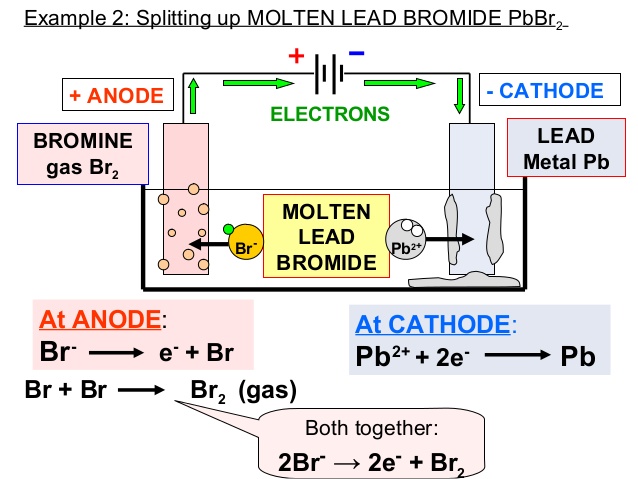
At the cathode (-) molten lead is formed:
· lead(II) ions (Pb2+) are attracted to the negative electrode
· the Pb2+ ions are forced to accept two electrons
· the ion-electron half equation for this reaction is:
Pb2+(l) + 2e- → Pb(l)
At the anode, gaseous bromine is evolved:
· bromide ions (Br-) are attracted to the positive electrode
· the bromide ions are forced to give away their extra electron to form bromine atoms
· the bromine atoms join up in pairs to form diatomic bromine molecules (Br2).
· the ion-electron half equation for this reaction is:
2Br- (l) → Br2(g) + 2e-
The products of this electrolysis are:
· lead metal at the negative electrode. The lead will form as a liquid at the bottom of the reaction vessel.
· bromine (Br2) at the positive electrode. The bromine appears as a brown gas at the positive electrode.
Overall equation for the electrolysis of molten lead bromide:
PbBr2(l) ==> Pb(l) + Br2(g)
If there is time, you may like to show that heating alone is insufficient to cause the lead(II) bromide to decompose.
Дата добавления: 2018-11-25; просмотров: 1521;
