FARADAY'S LAWS OF ELECTROLYSIS
The laws, which govern the deposition of substances (In the form of ions) on electrodes during the process of electrolysis, is called Faraday's laws of electrolysis. These laws given by Michael Faraday in 1833.
(1) Faraday's first law: It states that, “The mass of any substance deposited or liberated at any electrode is directly proportional to the quantity of electricity passed.”i.e., W 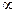 Q
Q
Where,
W = Mass of ions liberated in gm,
Q = Quantity of electricity passed in Coulombs = Current in Amperes (I) × Time in second (t)
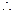 W
W 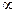 I × t or W = Z × I × t
I × t or W = Z × I × t
In case current efficiency (η) is given, then
W = Z × I × t × η/100
where, Z = constant, known as electrochemical equivalent (ECE)of the ion deposited.
When a current of 1 Ampere is passed for 1 second (i.e.,Q = 1), then, W = Z
Thus, electrochemical equivalent (ECE) may be defined as “the mass of the ion deposited by passing a current of one Ampere for one second (i.e., by passing Coulomb of electricity)”. It's unit is gram per coulomb.
Coulomb is the unit of electrical charge.
96500 Coulombs electrons = 1 mole electrons.
1 Coulomb = 6.023×1023/96500 = 6.85 × 1018 electrons,
or 1 electronic charge 1.6 × 10–19 Coulomb.
(2) Faraday's second law: It states that, “When the same quantity of electricity is passed through different electrolytes, the masses of different ions liberated at the electrodes are directly proportional to their chemical equivalents (Equivalent weights).” i.e.,
W1/W2 = E1/E2 or Z1It/Z2It or Z1/Z2 = E1/E2 ( 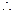 W = ZIt)
W = ZIt)
Thus the electrochemical equivalent (Z) of an element is directly proportional to its equivalent weight (E), i.e., E 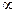 Z or E = FZ or E = 96500 × Z
Z or E = FZ or E = 96500 × Z
where, F = Faraday constant = 96500 C mol–1
So, 1 Faraday = 1F =Electrical charge carried out by one mole of electrons.
1F = Charge on an electron × Avogadro's number.
1F = e– × N = (1.602 × 10–19c) × (6.023 × 1023 mol–1)
Number of Faraday = number of electrons passed/6.023 × 1023
Дата добавления: 2018-11-25; просмотров: 628;
