APPLICATION OF ELECTROLYSIS
Electrolysis has wide applications in industries. Some of the important applications are, as follows,
1) Production of hydrogen by electrolysis of water.
2) Manufacture of heavy water (D2O).
3) The metals like Na, K, Mg, Al, etc., are obtained by electrolysis of fused electrolytes.
4) Non-metals like hydrogen, fluorine, chlorine are obtained by electrolysis.
5) In this method pure metal is deposited at cathode from a solution containing the metal ions Ag, Cu etc.
6) Compounds like NaOH, KOH, Na2CO3, KClO3, white lead, KMnO4 etc. are synthesised by electrosynthesis method.
7) Electroplating:The process of coating an inferior metal with a superior metal by electrolysis is known as electroplating.
Several processes of electrolysis are used in today's industry:
Electrorefining. The anode is the impure metal and any impurities are removed during the process of electrolysis when the metal travels from anode to cathode. During the electrorefining of metals, the cathode has a decomposition of pure metals from a solution containing the metal ion. For example copper is purified through electrolysis in order to be used for applications that require high electrical conductivity. During this process, the cathode is a pure piece of copper, while the anode is an impure piece of copper. The Cu2+ from the anode moves through a sulfuric acid-copper(II) sulfate solution into the cathode where it becomes solid copper. While this is occurring, the impurities are left at the bottom of the tank. This leftover residue is called anode mud. The electrolysis is carried out at 0.15 - 0.30V (low voltage) in order to make sure that Ag, Au, and Pt impurities are not oxidized while in the anode and become anode mud. Whereas most of the other components become oxides or hydroxides and form water-soluble species.
Electrosynthesis a method which uses electrolysis reactions to produce certain products. For example MnO2 needs to undergo electrolysis in order to be used for alkaline batteries. The solution for the electrosynthesis of MnO2is MnSO4in H2SO4. The anode is graphite, where Mn2+ is oxidized. While at the cathode, hydrogen is reduced from H+ to H2.
Overall Reaction: Mn2+(aq) + 2H2O(l)--> MnO2(s) + 2H+(aq) + H2(g)
Chloro-Alkali Process. Electrolysis of seawater which leads to the production of chlorine and the alkali, sodium hydroxide. There are 3 different methods in which these two components are produced: membrane cell, diaphragm cell, and mercury cell process.
Mercury Cell Process. Electrolysis of seawater in a mercury cell leads to the production of chlorine and sodium hydroxide at the same time. This method involves using mercury as the cathode and graphite as the anode. The mercury attracts either sodium or potassium cations and the mercury forms an amalgam with it. However when the amalgam is introduced to water it forms sodium hydroxide and hydrogen leaving the mercury to be reused later. The chlorine gas is left to form at the anode.
Diaphragm Cell Process. The diaphragm cell has Cl2 being released from the anode section, while there is H2 being released from the cathode section. If Cl2 manages to mix with NaOH, the Cl turns into other products. Therefore the diaphragm cell has a bigger amount of NaCl, and a smaller amount of solution in the cathode in order for the NaCl to come in contact with the other solution gradually, while simultaneously preventing backflow of NaOH.
Membrane Cell Process. This process is more efficient than others as it does not use mercury and does not require a significant amount of energy. Contains a cation-exchange membrane which is usually made from flourocarbon polymer. This membrane allows hydrated cations to pass in between the anode and cathode compartments, but does not allow the backflow of the ions, Cl- and OH-. This allows the sodium hydroxide produced to have less contamination by chloride ions.
Electroplating defined as the coating of an object with a thin layer of metal via electrolysis. It is also known as the deposition of a metal coating onto an object by putting a negative charge on it and putting it into a solution which contains a metal salt. The metal salt contains positively charged metal ions which are attracted to the negatively charged object and are reduced to a metal atom.
For instance, the electroplating of an object with copper, using copper(II) sulfate as the electrolyte, copper as the anode and the object to be electroplated as the cathode.
At the anode: Copper metal is oxidised to form copper ions. The copper ions enter the solution. Equation: Cu(s) -> Cu2+(aq) + 2e-
At the cathode: Copper(II) ions are reduced to form copper metal, which is deposited on the object. There is a net movement of copper from the anode to the cathode. Copper (II) sulphate solution remains unchanged.
Equation: Cu2+ (aq) + 2e- -> Cu (s)
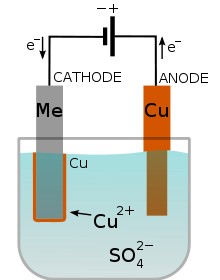
Electroplating is very important and have many uses, for instance to protect the surfaces of other metals, like nickel plating of iron to prevent iron from being oxidised and rusting, to make objects attractive, like electroplating of silver and gold on brass, and to repair machine parts. Sometimes, specific metals like nickel are used to prevent rust, while silver is used for utensils and trophies/metals.
Questions
1) Magnesium may be obtained commercially from sea water. During the last stage of this process, molten magnesium chloride undergoes electrolysis in a cell that contains an iron cathode and a graphite anode.
a) Why may iron be used to form the cathode but not the anode?
b) Draw a fully labelled diagram of an electrolytic cell that could be used to produce magnesium. Include equations.
2) Electrolysis of a concentrated copper (II) chloride solution produces copper (in solid form) and chlorine gas. When some metals are used as anodes, they may be oxidised in preference to water or the ions in solution? Platinum and graphite electrodes do not react.
3) A solution containing lead, magnesium and copper ions is electrolysed for a
long time.
a) What will be the first product formed at the cathode?
b) If the electrolysis is continued until all the ions responsible for the product in (a) are used up, what will be the next product observed at the cathode?
c) If the electrolysis is continued further until the product from (b) is observed to stop forming, what will be the third product formed at the cathode?
4) Predict the products formed from the electrolysis, using inert electrodes, of:
a) mmolten lead (II) chloride
b) a 1 M solution of lead (II) chloride.
5) Considering the three factors affecting selectivity discharge, explain what happens when a diluted solution of sodium iodide is electrolysed?
6) Explain what happens when copper (II) sulfate is used as an electrolyte and inert electrodes are used?
7) State what is observed when copper (II) sulfate is used as an electrolyte and inert electrodes are used?
8) Explain in terms of oxidation number what is being reduced or oxidised when Sodium Chloride is used as an electrolyte with inert electrodes.
9) Construct the equation to show the chemical changes during the electrolysis of molten sodium chloride?
10) Define electrolysis?
Test
1. Which of the following states of ionic compound are electrolytes?
A. Solid state
B. Solid and Molten state
C. Molten state
D. Aqueous and Molten state
2. Which of the following involves electrolysis?
A. Photosynthesis and Respiration
B. Purification of Copper and Sea Water
C. Purification of copper and extraction of reactive metals
D. Extraction of reactive metals and Respiration
3. Which of the following is not an inert electrode?
A. Carbon B. Copper C. Platinum D. Mercury
4. What ions are present in the electrolysis of aqueous Copper(II) Sulfate with Copper electrodes?
A. Copper(II) ions, Sulfate ions
B. Hydrogen ions, Oxygen ions, Copper(II) ions
C. Copper (II) ions
D. Hydrogen ions, Oxygen ions, Copper(II) ions, Sulfate ions
5. What happens during the electrolysis of a concentrated sodium iodide solution?
A. Sodium ion and hydrogen ion are discharged.
B. Hydrogen ion and hydroxide ion are discharged.
C. Sodium ion and iodide ion are discharged
D. Hydrogen ion and Iodide ion are discharged
6. Which of the following are common electroplating metals?
A. Chromium, Potassium, Sodium
B. Tin, Silver, Magnesium
C. Tin, Silver, Cromium
7. What happens during the electrolysis of a diluted sodium chloride solution.
A. Hydrogen ions and chlorine ions are discharged.
B. Hydrogen ions and hydroxide ions are discharged.
C. Sodium ion and chlorine ions are discharged.
D. Sodium ions and hydroxide ions are discharged.
8. What do "anode sludge" commonly contain?
A. Sodium, Glucose, Potassium
B. Gold, Silver, Impurities
C. Impurities, Silver, Sodium
D. Gold, Potassium, Oxygen
9. Non-conductors be electroplated?
A. True B. False
References
1. Petrucci, et al. General Chemistry: Principles & Modern Applications. 9th ed. Upper Saddle River, New Jersey: Pearson/Prentice Hall, 2007.
2. Kolbe, Hermann. The Electrolysis of Organic Compounds. Edinburgh : E. & S. Livingstone, 1947.
3. Stuart, A.T. "Electrolysis of water." HydrogenProduction 2001 May 13
4. All of the afformentioned "Outside Links" as well.
5. https://chem.libretexts.org/Core/Analytical_Chemistry/Electrochemistry/Electrolytic_Cells
6. http://freechemistryonline.com/electrolytic-cell.html
7. http://www.emedicalprep.com/study-material/chemistry/electrochemical-series-applications.html
Дата добавления: 2018-11-25; просмотров: 1086;
