AN ELECTROLYTIC CELL CONSTRUCTION
An electrolytic cell is obtained by connecting a source of direct current, DC into an electrolyte through electrodes. The direct current is provided by batteries whose positive end is connected by wire to the positive electrode (called anode) and negative end connected to the negative electrode (called cathode) as shown below:
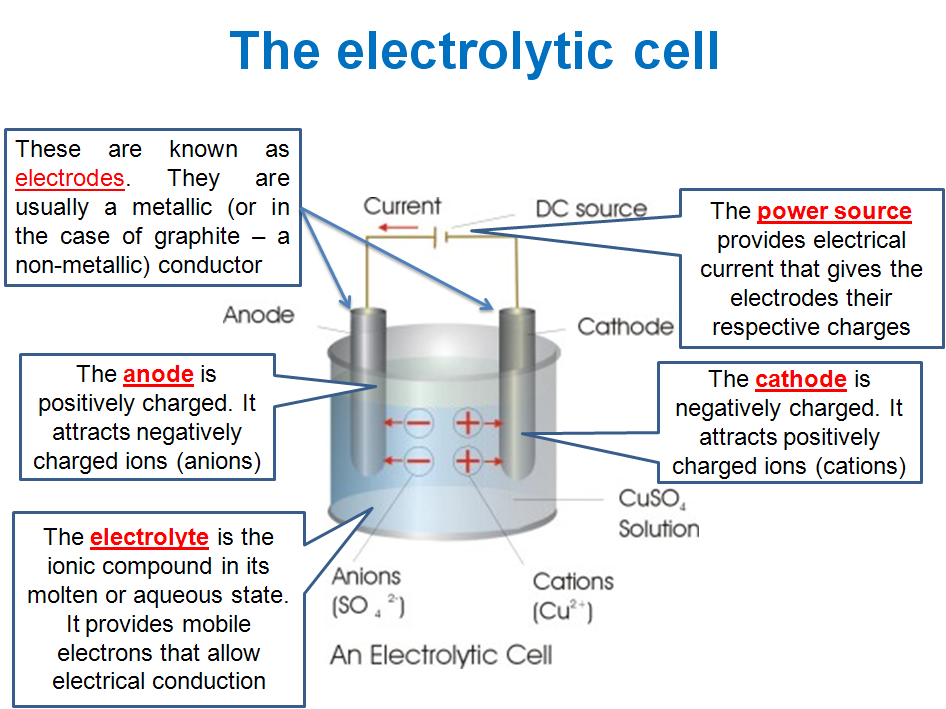
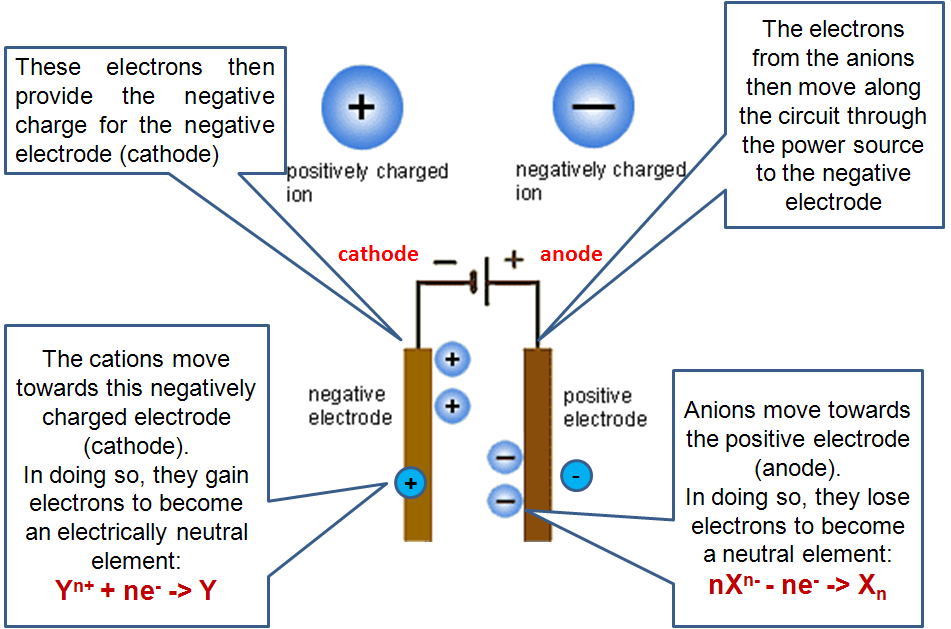
When the switch is on, electrons flow from the negative end of the batteries into the cathode. The concentration of electrons on the cathode causes the positive ions in solution to migrate to the cathode, while negative ions migrate to the anode.
At the cathode, one of the positive ions picks up electrons and become reduced, while at the anode, one of the negative ions loses the same number of electrons picked up at the cathode and become oxidized. The lost electrons are taking away through the wire, back to the batteries.
The bulb lights up, indicating that the circuit is complete, which means that the solution is a conductor of electricity. The process continues, with gradual reduction in the intensity of the light due to the decomposition and loss of the electrolyte.
Notice that conduction of electricity is due to the migration of opposite ions to the electrodes. The above set-up can be used to determine whether the solution of a substance is an electrolyte or not (solutions of non-electrolytes will not produce light in the bulb).
It can also be used to differentiate strong electrolytes from weak electrolytes (strong electrolytes will produce bright light, while weak electrolytes will produce dull light).
The chemical reactions that took place at both electrodes needed external electrical power to be applied before they would occur. This means they are non-spontaneous reactions.
Дата добавления: 2018-11-25; просмотров: 569;
