Electrolytic cell¢s structure
An electrochemical process is a chemical reaction that either causes or is caused by the movement of electrical current. These processes are a type of oxidation-reduction reaction in which one atom or molecule loses an electron to another atom or molecule. In electrochemical reactions, the atoms or molecules in the reaction are relatively far apart from each other compared to other reactions, forcing the electrons being transferred to travel a greater distance and thereby produce an electrical current. Many natural phenomena are based on electrochemical processes, such as the corrosion of metals, the ability of some sea creatures to generate electrical fields, and the workings of the nervous systems of humans and other animals. They also play an important role in modern technology, most prominently in the storage of electrical power in batteries, and the electrochemical process called electrolysis is important in modern industry.
In most of our discussions of chemical reactions, we have assumed that the reactants are in intimate physical contact with one another. Acid–base reactions, for example, are usually carried out with the acid and the base dispersed in a single phase, such as a liquid solution. With redox reactions, however, it is possible to physically separate the oxidation and reduction half-reactions in space, as long as there is a complete circuit, including an external electrical connection, such as a wire, between the two half-reactions. As the reaction progresses, the electrons flow from the reductant to the oxidant over this electrical connection, producing an electric current that can be used to do work. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell.
The cell includes:
· an anode, the electrode where oxidation takes place
· a cathode, the electrode where reduction takes place
· an electrolyte, to allow conduction of ions within the solution in each half cell
· a salt bridge or semipermeable membrane, to allow conduction of ions between half cells
· the external circuit that connects the two electrodes includes wires, a load, and meters.
There are two types of electrochemical cells: galvanic cells and electrolytic cells.
Voltaic (galvanic) cells are driven by a spontaneous chemical reaction that produces an electric current through an outside circuit. These cells are important because they are the basis for the batteries that fuel modern society. But they are not the only kind of electrochemical cell. The reverse reaction in each case is non-spontaneous and requires electrical energy to occur.
An electrolytic cell is an electrochemical cell in which the energy from an applied voltage is used to drive an otherwise nonspontaneous reaction. Such a cell could be produced by applying a reverse voltage to a voltaic cell like the Daniell cell.It is operate through electrolysis. Electrolysis is used to drive an oxidation-reduction reaction in a direction in which it does not occur spontaneously by driving an electric current through the system while doing work on the chemical system itself, and therefore is non-spontaneous.
Electrolytic cells, like galvanic cells, are composed of two half-cells – one is a reduction half-cell, the other is an oxidation half-cell. The direction of electron flow in electrolytic cells, however, may be reversed from the direction of spontaneous electron flow in galvanic cells, but the definition of both cathode and anode remain the same, where reduction takes place at the cathode and oxidation occurs at the anode. Because the directions of both half-reactions have been reversed, the sign, but not the magnitude, of the cell potential has been reversed.
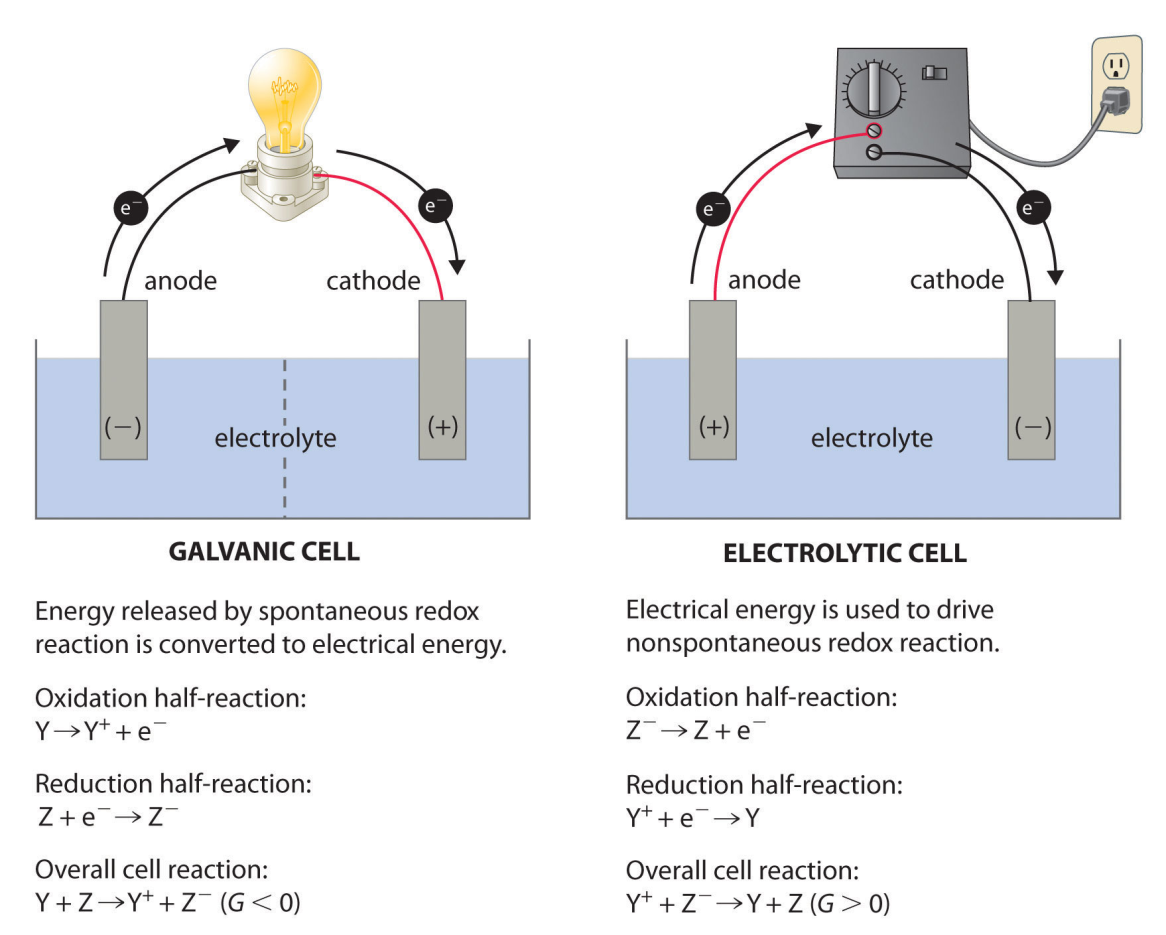
The main points of difference between an electrolytic cell and a galvanic cell (electrochemical cell) are:
| GalvanicCell | Electrolytic cell |
| A Galvanic cell converts chemical energy into electrical energy. | An electrolytic cell converts electrical energy into chemical energy. |
| Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. | The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. |
| The two half-cells are set up in different containers, being connected through the salt bridge or porous partition. | Both the electrodes are placed in a same container in the solution of molten electrolyte. |
| Here the anode is negative and cathode is the positive electrode. The reaction at the anode is oxidation and that at the cathode is reduction. | Here, the anode is positive and cathode is the negative electrode. The reaction at the anode is oxidation and that at the cathode is reduction. |
| The electrons are supplied by the species getting oxidized. They move from anode to the cathode in the external circuit. | The external battery supplies the electrons. They enter through the cathode and come out through the anode. |
Дата добавления: 2018-11-25; просмотров: 685;
