Carbon based materials: Fullerenes. The structure and its characteristics. Types of fullerenes. Mechanism of formation. Chemical properties. Applications.
Objectives:
Ø To describe the structure and the most important characteristics of fullerenes, their types, formation and properties.
Ø To give the most important applications.
Carbon is found in every corner of the universe. It is the most studied chemical element. Here on Earth, every single living thing contains carbon atoms. This atom forms many complex molecules, especially with hydrogen, oxygen, and nitrogen—millions and millions of organic compounds of great importance in our daily life. It is fundamental in biology and medicine, but also in the production of energy and the conservation of the environment.
For a long time, it was considered that pure carbon, at ambient temperature and pressure, existed in the form of two types of materials: graphite, which we use in the pencil mines, and diamond, crystalline cubic structure. Carbon atoms form very strong covalent bonds with other carbon atoms, which explains diamond’s strength.
Carbon is known for its large variety of allotropic forms, structural and topological characteristics which are the fundamental cause of several unique physical properties associated with carbon materials. It is not often that there are materials with very different characteristics formed by the same chemical element. During the last years were discovered more forms of carbon in nature.
In the 1970s, graphite intercalations were studied. They consist of very thin sheets of graphite, which can be superconducting, between layers of other materials.
Also in the 1970s polymers such as polyacetylene, which can be considered a very long chain of carbon atoms, with some bonds saturated with hydrogen, were studied intensively.
In the eighties, fullerene, a molecule of sixty carbon atoms (C60) and shaped like a soccer ball, was discovered in interstellar space (figure 1). Similar carbon molecules of similar size were synthesized.
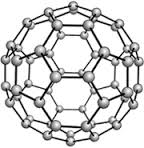 Fig. 1. The structure of the molecule C60, fullerene.
Fig. 1. The structure of the molecule C60, fullerene.
In the 1990s carbon nanotubes were discovered, very thin sheets of graphite rolled into a tube. Finally, at the beginning of this century, it was shown that graphite sheets with a thickness of only one atom could be isolated and manipulated: graphene.
Fig. 2. The versatile carbon
Today, we will start with the study of fullerenes, their properties and applications.
Fullerenes
Fullerenes are the third most stable form of carbon, after diamond and graphite. It has a cage-like fused-ring structure (truncated icosahedron) which resembles a soccer ball (football), made of hexagons and pentagons, with a carbon atom at each vertex of each polygon and a bond along each polygon edge.
The first fullerene was discovered in 1985 by Harold Kroto, James R. Heath, Sean O'Brien, Robert Curl, and Richard Smalley at Rice University. Kroto, Curl and Smalley were awarded the 1996 Nobel Prize in Chemistry for their roles in the discovery of buckminsterfullerene and the related class of molecules, the fullerenes. They have become popular among chemists, both for its structural beauty and its versatility for the synthesis of new compounds, due to their form like spheres, ellipsoids or cylinders. Spherical fullerenes are often called Bucky spheres and cylindrical ones are buckytubes or nanotubes due to their structural and mathematical resemblance to geodesic spheres, made by Buckminster Fuller. He also developed numerous inventions, mainly architectural designs, and popularized the widely known geodesic dome (Fig. 3).

Fig. 3. Geodesic dome
The most common form is called C60. It consists of 20 hexagonal and 12 pentagonal rings as the basis of icosahedral symmetry closed cage structure. As is shown in fig. 1, each carbon atom is bonded to three others and is sp2 hybridized. This molecule has two bond lengths - the 6:6 ring bonds can be considered "double bonds" and are shorter than the 6:5 bonds. It has 60 carbon atoms (Fig. 4) bonded together just like the hexagons and pentagons on a soccer ball (Fig. 1).
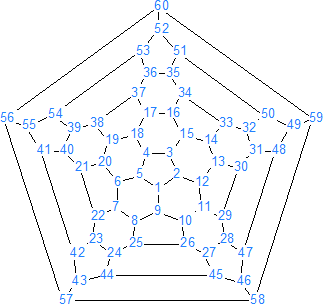
Fig. 4. Structure of C60 molecule.
Larger fullerenes have been found or synthesized, including C70, C76, C80, C84, C90, C96, …, even up to C540.
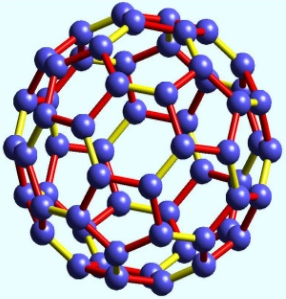
Fig. 5. Fullerene; Carbon atoms arranged in pentagons and hexagons
The bonding pattern of C60 fullerenes with yellow bonds representing double bonds and red bonds representing single bonds. The pentagonal rings contain only single bonds. Double bonds have a shorter bond length and lead to instability in the pentagonal rings (Fig. 5).
The limitations on double bonds locations lead to poor delocalization of electrons, increasing the molecule´s reactivity.
C60 is not "super aromatic" as it tends to avoid double bonds in the pentagonal rings, resulting in poor electron delocalization. As a result, C60 behaves like an electron deficient alkenes and reacts readily with electron rich species. The geodesic and electronic bonding factors in the structure account for the stability of the molecule. They are macromolecules with a highly symmetrical and stable structure.
Since the discovery of the fullerenes in 1985, their structural variations have evolved far beyond the different groups themselves. Examples of fullerenes include:
Types of fullerene
ü Bucky ball clusters: smallest member is C20 (unsaturated version of dodecahedrane) and the most common is C60. Other examples include C26, C36, C50, C70, C72, C76, C80, C82, and C84.
ü Nanotubes: hollow tubes of very small dimensions, having single or multiple walls; potential applications in electronics industry.
ü Megatubes: larger in diameter than nanotubes and prepared with walls of different thickness; potentially used for the transport of a variety of molecules of different sizes.
ü Polymers: chain, two-dimensional and three-dimensional polymers are formed under high-pressure high-temperature conditions.
ü Nano"onions": spherical particles based on multiple carbon layers surrounding a buckyball core.
ü Linked "ball-and-chain" dimers: two buckyballs linked by a carbon chain.
Дата добавления: 2018-11-25; просмотров: 823;
