Properties of fullerenes
Fullerenes have fascinating new chemical, physical, and biological properties. By manipulating the fullerenes can modify their properties to make new materials. The manipulation of the fullerenes can be done by replacing some carbon atoms with atoms of another material and/or by introducing nano molecules of other materials into their structure.
ü Physical properties
It is an extremely strong and resistant carbon macromolecule; its density is 1.72 g /cm3 and its diameter varies between 7 and 15 Å. It resists extraordinary pressures, up to 3 atm and has great tensile strength. There are some others physical constants such as:
- Thermal conductivity (300 K): 0.4 W m-1 K-1
- Electrical conductivity: 1,7.10-7 Cm
- Boiling temperature: 1180 0C
Fullerenes are not highly reactive due to the stability of graphite bonds, and are also very poorly soluble in most solvents. Common solvents for fullerenes include toluene and carbon disulfide. The solutions of pure buckminsterfullerene have an intense purple color. Fullerene is the only allotropic form of carbon that can be dissolved. Researchers have been able to increase their reactivity by attaching active groups to the surfaces of fullerenes.
ü Chemical properties
1. Reactions of addition: there is a lot of addition reactions of fullerenes, but we will study only some examples.
1.1. Halogenation
Under various conditions a vast number of halogenated derivatives of C60 can be produced.
At the beginning, we said that the limitations on double bonds locations lead to poor delocalization of electrons, increasing the molecule´s reactivity. C60 tends to avoid double bonds in the pentagonal rings, resulting in poor electron delocalization. As a result, C60 behaves like an electron deficient alkenes and reacts readily with electron rich species like fluorine and chlorine.
Simple inorganic derivatives of fullerenes are fluorides. The most apparent kinetic mechanism is based on the fact that after joining the first two fluorine atoms at double bond, the preferred reactive centers become double bonds in two contiguous six-membered cycles (Fig. 6)
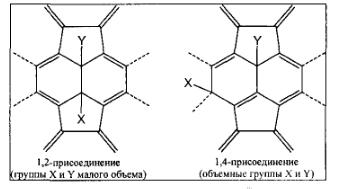
Fig. 6. Fragments of the chemical structures for the products of attaching two ligands to the fullerene C60 in 1,2 and 1,4 – positions.
Therefore, we can assume that the consecutive addition of fluorine atoms can proceed via competing routes S and T, shown in figure 6.
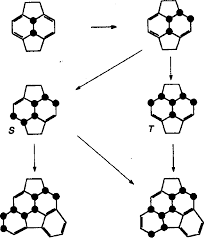
Fig. 6. The proposed scheme sequential filling of the fullerene sphere by atoms of fluorine.
As a result of fluorination, we can get fragments of C60F2, C60F4, C60F6, C60F8
The process of chlorination of fullerene C60 with ICl/ICl3 can be represented in the following
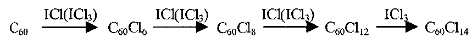
1.2. Hydrogenation
C60 can undergo addition with hydrogen to give polyhydrofullerenes as fallow:

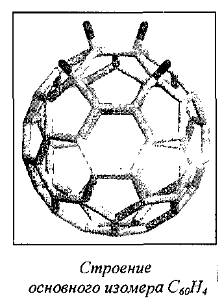
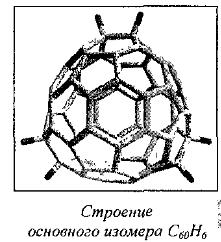
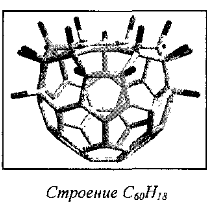
The simplest derivative is C60H2:
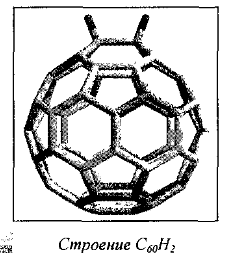
Under certain conditions, can be obtained other isomers:
2. Endohedral fullerenes
One of the unique properties of fullerene molecules is an ability to sign inside of their carbon skeleton atoms and molecules. Thus, the resulting compounds are called endofullerenes. They are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. This property of fullerenes to create endohedral compounds can be used to create molecular containers.
Among the compounds, which are usually referred to as endohedral fullerenes, there are two main groups. The first group contains within the carbon skeleton one or more metal atoms. The second group includes compounds endohedral fullerenes with atoms of inert gases and nitrogen.
The first lanthanum C60 complex was synthesized in 1985 and called La@C60. The @ (at sign) in the name reflects the notion of a small molecule trapped inside a shell.
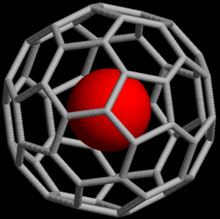
Fig. 4. Rendering of a buckminsterfullerene containing an atom (M@C60).
In a traditional chemical formula notation, a buckminsterfullerene (C60) with an atom (M) was simply represented as MC60 regardless of whether M was inside or outside the fullerene. To allow for more detailed discussions with minimal loss of information, a more explicit notation was proposed in 1991, where the atoms listed to the left of the @ sign are situated inside the network composed of the atoms listed to the right. The example above would then be denoted M@C60 if M were inside the carbon network. A more complex example is K2(K@C59B), which denotes "a 60-atom fullerene cage with one boron atom substituted for a carbon in the geodesic network, a single potassium trapped inside, and two potassium atoms adhering to the outside.
The choice of the symbol has been explained by the authors as being concise, readily printed and transmitted electronically (the at sign is included in ASCII, which most modern character encoding schemes are based on), and the visual aspects suggesting the structure of an endohedral fullerene.
Дата добавления: 2018-11-25; просмотров: 1095;
