Properties of metals
Almost all metals have some common properties: a metallic luster, the structure of the crystal lattice, the ability in chemical reactions to show properties reductant and not to oxidize. In chemical reactions ions of dissolved metals form salts in the interaction with the acid, by interaction with water (depending on the activity of the metal) form alkali or base.
Below are the physical properties of metals:
Physical State:Metals are solids at room temperature with the exception of mercury and gallium, which are liquids at room temperature.
Lustre:This means they are shiny. Metals have the quality of reflecting light from its surface and can be polished e.g., gold, silver and copper. Photons of light do not penetrate very far into the surface of a metal and are typically reflected, or bounced off, the metallic surface.
Malleability: Metals have the ability to withstand hammering and can be made into thin sheets known as foils. The delocalised electrons in the 'sea' of electrons in the metallic bond, enable the metal atoms to roll over each other when a stress is applied.
Ductility:Metals can be drawn into wires. 100 gm of silver can be drawn into a thin wire about 200 meters long.
Hardness:All metals are hard except sodium and potassium, which are soft and can be cut with a knife.
Sonority:They produce sounding noise when collide.
Valency: Metals have 1 to 3 electrons in the outermost shell of their atoms.
Conduction:Metals are good conductors because they have free electrons. The delocalised electrons are free to move in the solid lattice. These mobile electrons can act as charge carriers in the conduction of electricity or as energy conductors in the conduction of heat.

Silver and copper are the two best conductors of heat and electricity. Lead is the poorest conductor of heat. Bismuth, mercury and iron are also poor conductors.
Density:Metals have high density and are very heavy. Iridium and osmium have the highest densities where as lithium has the lowest density.
Melting and Boiling point:Metals have high melting and boiling point. Tungsten has the highest melting point where as silver has low boiling point. Sodium and potassium have low melting points.
Electropositive Character:Metals are elements that have a tendency to lose electrons and form cations. They normally do not accept electrons.
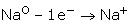
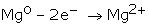
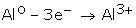
To summarize: metals are electropositive in nature, lustrous, malleable, ductile, good conductors of heat and electricity and generally form basic or amphoteric oxides with oxygen.
Below are the chemical properties of metals: Metals are usually inclined to form cations through electron loss. An example is the reaction with oxygen in the air to form oxides over various timescales (iron rusts over years, while potassium burns in seconds). The transition metals (such as iron, copper, zinc, and nickel) are slower to oxidize because they form a passivating layer of oxide that protects the interior. Others, like palladium, platinum, and gold, do not react with the atmosphere at all. Some metals form a barrier layer of oxide on their surface, which cannot be penetrated by further oxygen molecules. As a result, they retain their shiny appearance and good conductivity for many decades (like aluminium, magnesium, some steels, and titanium).
l) Reaction with oxygen: Metals when heated in air, react with oxygen to form oxides.
2Cu + O2 = 2CuO – Q
4Al + 3O2 = 2Al2O3 – Q
4Na + O2 = 2Na2O + Q
The most reactive metals as potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air.
Metals from aluminium to copper in the activity series of metals, react slowly when heated in air to form the metal oxides. Aluminium is the fastest and copper is the slowest of them.
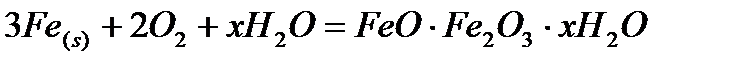 Iron metal does not burn in dry air even on strong heating. In moist air, iron is oxidized to give rust:
Iron metal does not burn in dry air even on strong heating. In moist air, iron is oxidized to give rust:
Gold and platinum do not react with oxygen in air.
Metal oxides are basic. Some of them dissolve in water to form alkali hydroxide and turns red litmus blue:
Na2O(s) + H2O => 2NaOH(aq) CaO(s) + H2O => Ca(OH)2(aq)
sodium sodium calcium calcium
oxide hydroxide oxide hydroxide
2) Reaction with water: Those metals staying above hydrogen in electrochemical series react with cold water or steam to produce hydrogen:
1) 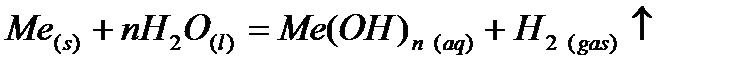 Active metals at room temperature are formed hydroxide:
Active metals at room temperature are formed hydroxide:
2) 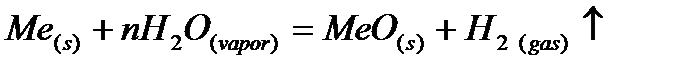 Medium active metals at high temperature with steam are formed oxide:
Medium active metals at high temperature with steam are formed oxide:
3) Tin, lead, copper, silver, gold and platinum do not react with water or steam.
3) Reaction with chlorine: Metals react with chlorine to form corresponding chlorides.
Na(s) + Cl2(g) => NaCl(s) Mg(S) + Cl2(g) => MgCl2(s)
sodium chloride magnesium chloride
4) Reaction with acids: Most of the metals like potassium, sodium, lithium and calcium react violently with dilute H2SO4 and dilute HCl, forming the metal salt (either sulfate or chloride) and hydrogen gas:
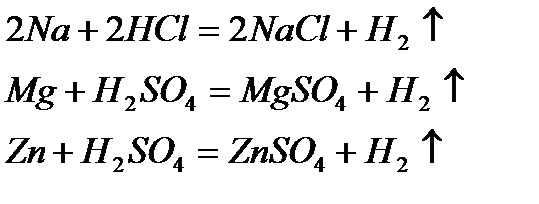
Zinc with dilute sulfuric acid is often used for the laboratory preparation of hydrogen. The reaction is slow at room temperature, but its rate can be increased by the addition of a little copper (II) sulfate. Zinc displaces copper metal, which acts as a catalyst.
Metals below hydrogen (copper, silver, gold and platinum), will not react with dilute acids. They cannot displace hydrogen from the non-metal anion.
Hydrogen gas is not produced when metals react with nitric acid (HNO3) but magnesium and manganese metals react with very dilute nitric acid to form hydrogen gas.
5) Reaction of Metals with Concentrated Acids: HNO3 and H2SO4
Hydrogen gas is not evolved when metals react with nitric acid (HNO3) because it is a strong oxidising agent and it oxidises the H2 produced to water and is itself reduced to nitrogen dioxide:
1) With active metals:
Mg + HNO3(dilut) = Mg(NO3)2 + H2O + NH3 (NH4NO3)
Mg + HNO3(conc) = Mg(NO3)2 + 4H2O + 2N2O
2) With passive metals:
Cu + HNO3(dilut) = Cu(NO3)2 + H2O + NO
3Cu + 8HNO3(conc) = 3Cu(NO3)2 + 4H2O + 2NO2
• Reaction with concentrated sulfuric acid:
Me + H2SO4 (conc) = MeSO4 + H2O + (H2S, S, SO2)
Fe and Al will not react with conc H2SO4 acid, they are passivated.
6) Reaction with hydrogen:Some of the reactive metals like sodium, potassium, calcium etc. combine with hydrogen to produce metal hydrides.
2Na(s) + H2(g) => 2NaH(s) Ca(s) + H2(g) => CaH2(s)
sodium hydride calcium hydride
All metals are not equally active. Some of them are more active while some of them are less active. Metal which can easily remove electron to gain positive charge is an active metal; while less active metal does not lose electron easily. Activity of metal is compared with oxygen, water and acid. As all elements do not react with these reagents, substitution reactions are used to measure reactivity of metals e.g. more reactive metals can displace less reactive metals from their salt solution.
From experimental results, metals can be arranged in descending order of their activity which is known as activity series of metals. Metals on left hand side of hydrogen are more reactive than those on right hand side of hydrogen.
| Metal | Symbol | Reactivity |
| Lithium | Li | displaces H2 gas from water, steam and acids and forms hydroxides |
| Potassium | K | |
| Strontium | Sr | |
| Calcium | Ca | |
| Sodium | Na | |
| Magnesium | Mg | displaces H2 gas from steam and acids and forms hydroxides |
| Aluminum | Al | |
| Zinc | Zn | |
| Chromium | Cr | |
| Iron | Fe | displaces H2 gas from acids only and forms hydroxides |
| Cadmium | Cd | |
| Cobalt | Co | |
| Nickel | Ni | |
| Tin | Sn | |
| Lead | Pb | |
| Hydrogen gas | H2 | included for comparison |
| Antimony | Sb | combines with O2 to form oxides and cannot displace H2 |
| Arsenic | As | |
| Bismuth | Bi | |
| Copper | Cu | |
| Mercury | Hg | found free in nature, oxides decompose with heating |
| Silver | Ag | |
| Paladium | Pd | |
| Platinum | Pt | |
| Gold | Au |
The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement reactions and ore extraction. It can be used to predict the products in similar reactions involving a different metal.
The activity series is a chart of metals listed in order of declining relative reactivity. The top metals are more reactive than the metals on the bottom.
6) Displacement reaction:The reactivity series can be used to predict if a metal will react with a metal compound. If the metal is more reactive than the metal in the compound, it pushes out, or displaces, the less reactive metal from its compound. A displacement reactionis one where a more reactive metal will displace a cation of less reactive metal from a compound (salt, oxide):
Mg(s) + CuSO4(l) => MgSO4(l) + Cu(s)
Mg + ZnO => MgO + Zn
Дата добавления: 2018-11-25; просмотров: 1108;
