Categories of metals
Categories of various metals
Metals account for about two thirds of all the elements and about 24% of the mass of the planet. Metals have useful properties including strength, ductility, high melting points, thermal and electrical conductivity, and toughness.
Traditionally, the term metal (from the Greek word “metallon”) has been applied to a chemical element that has a shiny surface and is a good conductor of heat and electricity. These properties, however, can vary from one metal to the next. More recently, chemists have recognized that the main distinguishing features of a metal are (a) the ability of its atoms to lose some of their outermost electrons to form cations, and (b) the bonding of its atoms by what are called metallic bonds.
Of the 105 known elements (as of 1974), 83 were metals and only 22 nonmetals. Metals form one of three groups of elements – the other two being nonmetals and metalloids.
These groups are distinguished by their ionization and bonding properties. On the periodic table, a diagonal line drawn from boron (B) to polonium (Po) separates the metals from the nonmetals. Elements on this line are metalloids, sometimes called semi-metals; elements to the lower left are metals; elements to the upper right are nonmetals. In nature, nonmetals are more abundant than metals, but most elements in the periodic table are metals. Some well-known metals are aluminum, calcium, copper, gold, iron, lead, magnesium, platinum, silver, titanium, uranium, and zinc.
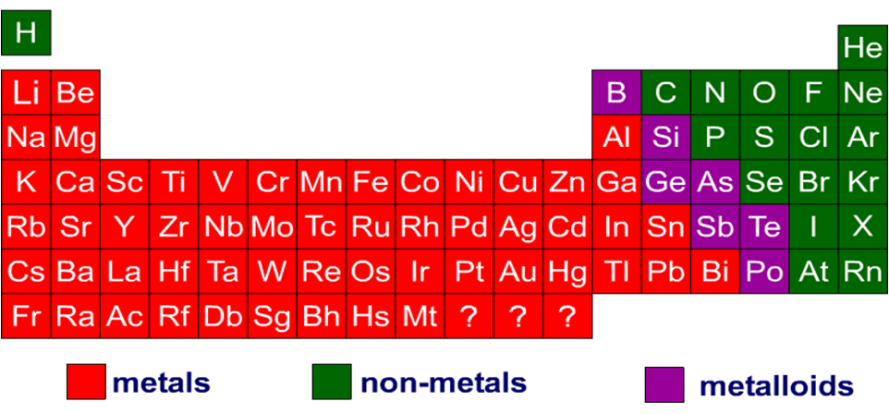
The properties of any element are defined by the number of electrons present in their valence shell. In case of metals, the outer shell contains 1-3 electrons, whereas the outer shell of nonmetals contains 4-8 electrons. Their configuration imparts them the chemical and physical properties they possess.
Categories of metals
In alchemy, the term base metal was used as a designation for common and inexpensive metals, to draw a contrast with precious metals such as gold and silver. A long-cherished goal of the alchemists was the transmutation of base metals into precious metals.
In chemistry today, the term base metal is used informally to refer to a metal that oxidizes or corrodes relatively easily and reacts variably with dilute hydrochloric acid (HCl) to form hydrogen. Examples include iron, nickel, lead, and zinc. Copper, too, is considered a base metal because it oxidizes relatively easily, although it does not react with HCl. Metals that resist oxidation or corrosion are called noble metals, which also tend to be precious metals.
A precious metal is a rare metallic chemical element of high, durable economic value. The best-known precious metals are gold and silver. Although both have industrial uses, they are better known for their uses in art, jewelry, and coinage. Other precious metals include the platinum group metals: ruthenium, rhodium, palladium, osmium, iridium, and platinum, of which platinum is the most widely traded. Plutonium and uranium may also be considered precious metals.
Chemically, the precious metals are less reactive than most elements. They have high luster and higher melting points than other metals. Historically, precious metals were important as currency, but are now regarded mainly as investment and industrial commodities. Investments in gold and silver are often regarded as a hedge against inflation and economic downturn.
Thus, there are metals alkaline, alkaline earth black, color, lanthanides (or rare earth metals - related to chemical properties of alkaline earth metals), actinoid (most of them are radioactive elements), and noble and platinum metals.
1) Alkali metals(group IA): Li, Na, K, Rb, Cs, Fr
2) Alkali earth metals(group IIA): Be, Mg, Ca, Sr, Ba, Ra
3) Transition metals(Group 3 – 12, d-elements): Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ag, Cd, Os, Hg, Pt, Au, W
• Iron Triad (Group 8, 9,10): Fe, Co & Ni = They create the magnetic field
• Coinage Metals (Group 11): Cu, Ag, & Au = They are used to make coins.
4) Post-transition metals:Al, In, Ga, Sn, Tl, Pb, Bi, Po
Lanthanides
Actinides
7) Elements which are possibly metals: meitnerium, darmstadtium, roentgenium, ununtrium, ununpentium, livermorium, ununseptium
8) Elements which are sometimes considered metals: Ge, As, At, Sb
Metal structure
All metals have similar properties BUT, there can be wide variations in melting point, boiling point, density, electrical conductivity and physical strength.
To explain the physical properties of metals like iron or sodium we need a more sophisticated picture than a simple particle model of atoms all lined up in close packed rows and layers, though this picture is correctly described as another example of a giant lattice held together by metallic bonding.
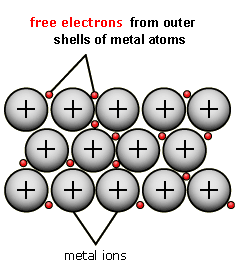 A giant metallic lattice – the crystal lattice of metals consists of ions (NOT atoms) surrounded by a 'sea of electrons' that form the giant lattice (2D diagram above right).
A giant metallic lattice – the crystal lattice of metals consists of ions (NOT atoms) surrounded by a 'sea of electrons' that form the giant lattice (2D diagram above right).
The outer electrons (–) from the original metal atoms are free to move around between the positive metal ions formed (+).
These “free” or “delocalized” electrons from the outer shell of the metal atoms are the 'electronic glue' holding the particles together.
There is a strong electrical force of attraction between these free electrons (mobile electrons or sea of delocalised electrons) (–) and the immobile positive metal ions (+) that form the giant lattice and this is the metallic bond.
Metallic bonding is not directional like covalent bonding, it is like ionic bonding in the sense that the force of attraction between the positive metal ions and the mobile electrons acts in every direction about the fixed (immobile) metal ions of the metal crystal lattice, but in ionic lattices none of the ions are mobile. It is a big difference between a metal bond and an ionic bond.
The strong metallic bonding generally results in dense, strong materials with high melting and boiling points.
Дата добавления: 2018-11-25; просмотров: 1046;
