CLASSIFICATION AND NOMENCLATURE OF ENZYMES
Modern classification and nomenclature of enzymes elaborated by the Commission on Enzymes of the International Union of Biochemistry and approved at the V International Congress of biochemistry in 1961 in Moscow.
The classification is based on three principles:
1. The chemical nature of the enzyme.
2. The chemical nature of the substrate.
3. Type of catalyzed reaction.
According to modern classification, the enzymes are divided into six major classes.
1. Oxidoreductases are enzymes that catalyze oxidation-reduction reactions:
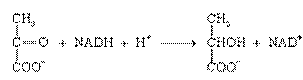
piruvate lactate
Aerobic dehydrogenase, or oxidase, catalyzes the transfer of protons (or electrons) directly to the oxygen.
Anaerobic dehydrogenase accelerates the transfer of protons (electrons) to an intermediate substrate, but not on oxygen.
The most common oxidoreductases contain as an active group nicotinamide adenine dinucleotide NAD+. Pyridine enzymes also contain as coenzyme nicotinamide adenine dinucleotide phosphate (NADP+). Coenzymes of oxidoreductases are also flavoproteins (FP) - flavin mononucleotide, FMN, and flavin adenine dinucleotide, FAD.
2. Transferases are enzymes that catalyze reactions of intermolecular transfer of various atoms and groups of atoms.
Phosphotransferases carry residue of phosphoric acid. Phosphate esters of organic compounds have increased chemical activity. ATP is a donor of phosphate group in most cases.
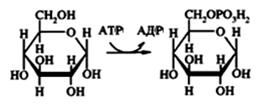
Aminotransferases accelerate reaction of amino group transport from the amino acids to α-keto acids.
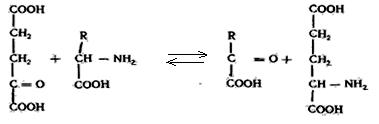
Proteinases accelerate the transfer of phosphate residue from ATP to proteins, altering their biological activity.
Glycosyl transferases accelerate transfer of glycosyl residues.
Acyltransferases catalyze the transfer of acyl groups (carboxylic acid residues).
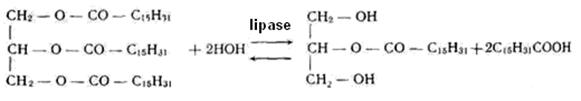
3. Hydrolases catalyze splitting of the intramolecular bonds of organic compounds with the participation of water. They are named in the form of "substrate-hydrolase."
Esterases catalyze the hydrolysis of the esters of alcohols with organic and inorganic acids. For example, lipase accelerates hydrolysis of triacylglycerols (fats):
Phosphatases catalyze the hydrolysis of phosphoric esters:
glucose-6-phosphate + H2O ® glucose + H3PO4
Glycosidases catalyze the hydrolysis of glycosides. Amylases are the most famous of glycosidases, acting on polysaccharides.
Peptidhydrolasesaccelerate the hydrolysis of peptide bonds in proteins and peptides.
4. Lyases are enzymes that catalyze the breaking of the C-O, C-C, C-N and other bonds, as well as the reversible reaction of detachment of the different groups of substrates by non-hydrolytic way, or addition - elimination. These reactions are accompanied by the formation of the double bonds and the releasing of the simplest products, such as CO2, H2O, NH3, and so on.
Carbon-carbon-lyase (decarboxylase):
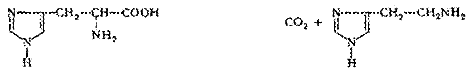
5. Isomerases are enzymes that catalyze the interconversion of structural, optical and geometric isomers. Mutarotase accelerates the conversion reaction of α-D-glucopyranose in the β-D-glucopyranose:

6. Ligases (synthetases) are enzymes that catalyze the synthesis of organic compounds from two molecules with the decay energy of ATP or other substances.
One of the most important is the pyruvate carboxylase:
CH3-CO-COOH + CO2 ® HOOC-CH2-CO-COOH
The International Commission has prepared Enzymes Classification (EC). Code of each enzyme contains four numbers separated by points. The first digit indicates the class number. The second digit indicates the subclass and characterized the type of substrate. For example, for transferases it indicates the nature of a transferred group, for the hydrolases it point to the type of hydrolyzed bond. The third digit specifies the nature of compounds or groups participating in the reaction. The fourth digit is number of the enzyme in the sub subclass.
An example: Pepsin - peptide-peptide hydrolase, EC 3.4.4.1.
Дата добавления: 2018-09-24; просмотров: 1281;
