Проблема регенерации сердечной мышечной ткани
(статьи)
+
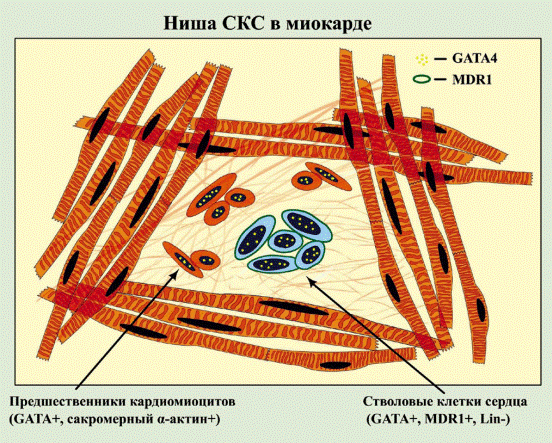
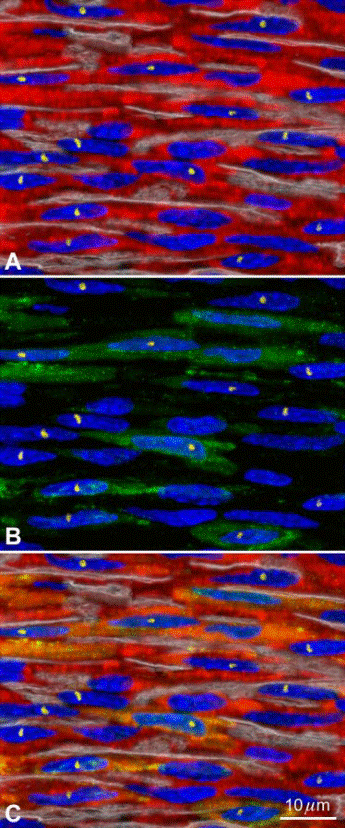
FIG. 4. Myocardial regeneration by BMCs. A: newly formed myocytes (troponin I; red) created by the injection of mouse male EGFP
tagged c-kit positive BMCs in a female infarcted mouse heart. Nuclei arestained by PI (blue). Laminin defines the boundaries of the cells (white
lines). The male phenotype of the regenerated myocytes is demonstrated by the presence of the Y chromosome in nuclei (yellow dots). B: a
significant fraction of new myocytes expresses EGFP in the cytoplasm (green). C: the merge of A and B shows the colocalization of troponin Iand EGFP (yellow-green) in the male myocytes.
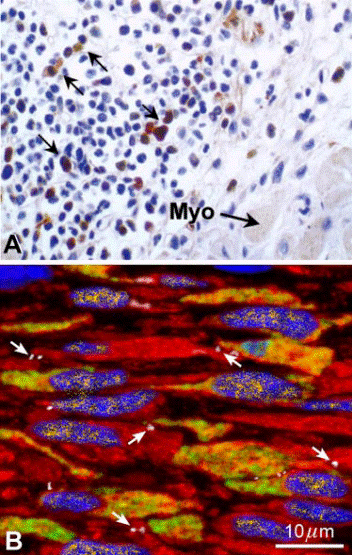
FIG. 5. BMC transdifferentiation in the infarcted heart: light microscop histochemistry and confocal microscopy immunofluorescence. A comparison is made between a section of 5–6 _m in thickness examineby light microscopy (A) obtained from Reference 324 and optical sections
of 0.5 _m analyzed by confocal microscopy (B). The image in Awas used to demonstrate the lack of transdifferentiation of BMCs intomyocytes. Conversely, the image in B documents the ability of BMCs toadopt the cardiomyogenic fate. The brown cells in A should correspond
to EGFP-positive cells that were not stained by any myocyte marker and were considered adequate for the unequivocal demonstration of thе absence of BMC transdifferentiation. There is no double staining in this preparation for EGFP and myocyte cytoplasmic proteins. In contrast, B
illustrates newly formed myocytes, _400 _m3 in volume, that are positive for EGFP (yellow green). These cells express GATA-4 in their nuclei (yellow), and connexin43 is detected at the cell boundary (white, arrows). Focal depth in A is 8% and in B is 12.5-fold higher, 100%.
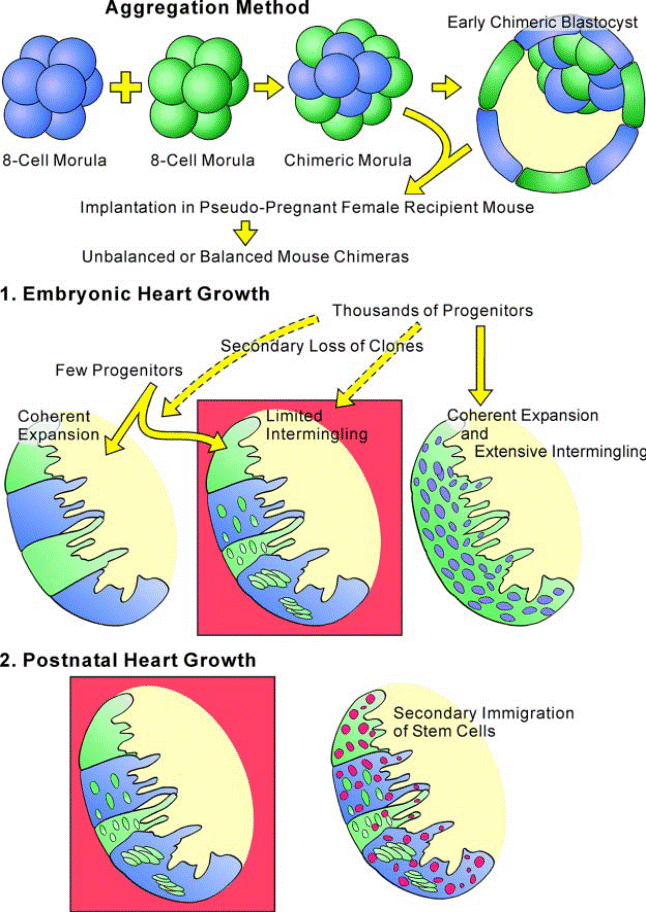
FIG. 8. Heart histogenesis and mouse chimeras. The combination of two 8-cell morulas of different genetic origin (blue _ LacZ; green _ EGFP) gives rise to a chimeric morula that evolves into a chimeric blastocyst. The implantation of the early chimeric blastocyst in pseudopregnant
mice results in balanced (equal parental contribution) or unbalanced (unequal parental contribution) mouse chimeras. By this approach, that is termed aggregation method, the number of progenitor cells that contribute to the development of the heart or other organs can be determined. The heart of these chimeric mice in which the two morulas carry either EGFP or LacZ originates from a single progenitor cell through a mechanism of coherent expansion of clones. In this case, patchy areas of the heart will be _-gal positive (blue) and others EGFP
positive (green). However, intermingling of clonogenic cells occurs and foci of _-gal-positive cells (blue) are found in EGFP clonal patches (green) or vice versa.
Limited intermingling of clonogenic cells and their progeny interferes partly with the coherent expansion of clonal patches. This pattern of embryonic heart growth is shown within the red box. The possibility that this model of cardiac growth is mediated by a large number of progenitors
would require a massive loss of clones early in development, a phenomenon that cannot be excluded. If thousands of progenitors were implicated in embryonic cardiac growth, a mechanism of coherent expansion together with extensive intermingling of clonogenic cells
would lead to a dispersed pattern of cardiomyocyte formation illustrated in the scheme. Importantly, the persistence of the embryonic clonal patches with small, intermingled
foci in the adult myocardium excludes that new clonal regions (red foci) contribute to the growth of the heart postnatally as graphically represented in the red box.
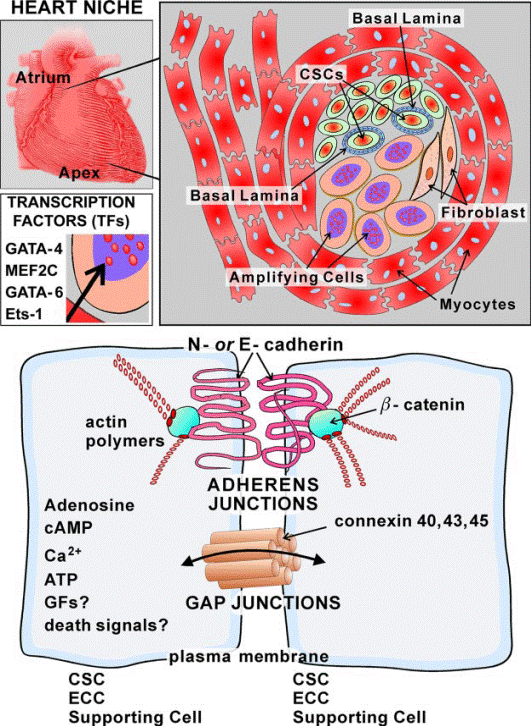
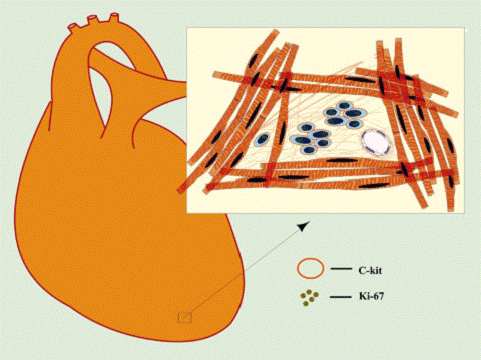
FIG. 9. Schematic representation of CSC niches. Cardiac niches are prevalently located in the atria and apex and consist of differentiated myocytes that surround clusters
of CSCs and highly dividing amplifying cells. The amplifying cells are committed cells and express transcription factors of the cardiac (GATA-4), myocyte (MEF2C), smooth muscle cell (GATA-6), and endothelial cell (Ets1) lineages. The interaction among CSCs, early committed
cells (ECCs), and supporting cells occurs via junctional proteins (cadherins and connexins).
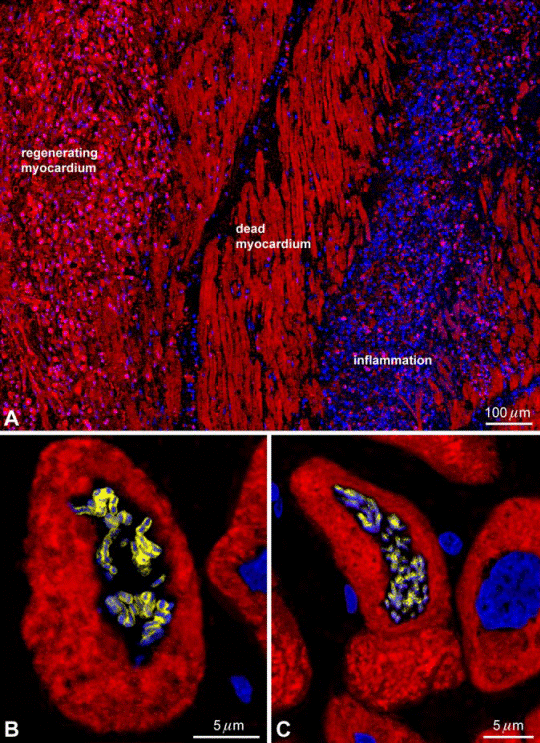
FIG. 10. Myocardial regeneration in the humanheart. A: an area of spontaneous formation of myocytes(_-sarcomeric actin, red) is present in proximity of dead, infarcted myocardium and a region of inflammatory reaction. B and C: metaphase chromosomes (PI, blue) are apparent in two small dividing myocytes (cardiac myosin heavy chain, red) in the heart of patients affected by chronic aortic stenosis. [Modified from Urbanek et al. (497).]
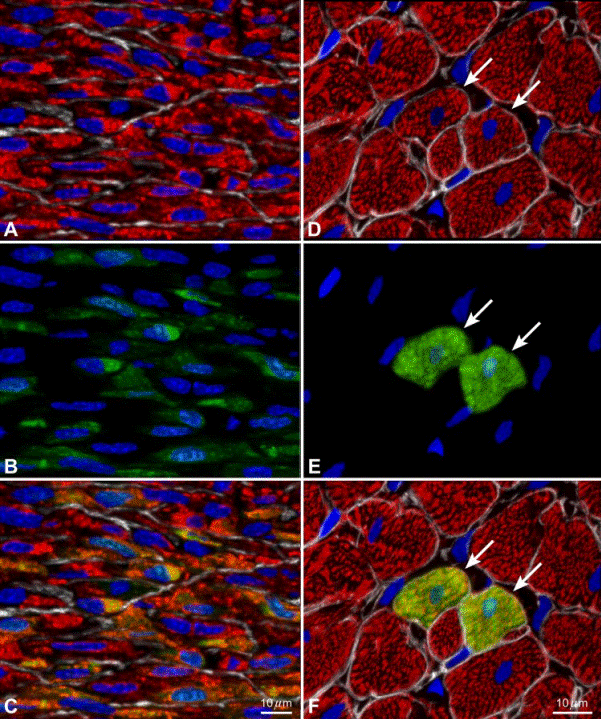
FIG. 13. Myocardial regeneration after infarction in rats. A–F: the local intramyocardial injection of EGFP-tagged CSCs in syngeneic rats acutely after permanent coronary artery occlusion results in the formation of new myocardium. A–C: in the infarcted region, the regenerated
myocytes are small (A; _-sarcomeric actin, red) and consistently show EGFP in the cytoplasm (B; green). C: the merge of A and B. The regenerated myocytes express both _-sarcomeric actin and EGFP (yellow-green). These myocytes are _700 _m3 in volume and, because of their size, cannot be the product of cell fusion. Nuclei are labeled by PI (blue). The localization of laminin at the periphery of the developing myocytes is illustrated by white lines. D–F: two newly
formed myocytes (arrows) are detected within the surviving myocardium of the border zone. They are positive for _-sarcomeric actin (D; red) and EGFP (E; green). F: the merge of D and E. The new myocytes are indistinguishable from the adjacent myocytes, _20,000 _m3 in volume.
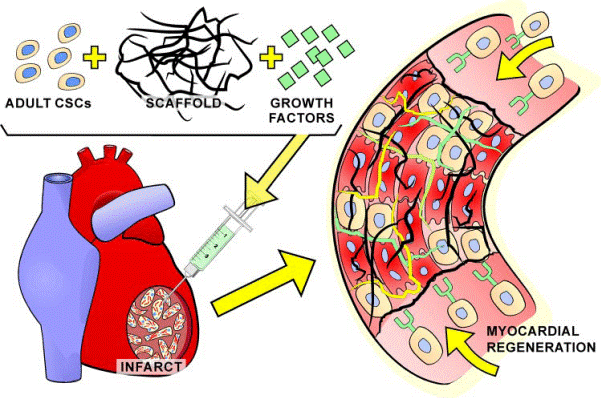
FIG. 15. Tissue engineering and stem cells. The injection of a self-assembling bioengineered scaffold loaded with adult CSCs and selective growth factors may enhance myocardial regeneration and orderly organization of parenchymal cells and vascular structures. Moreover,
the presence of growth factors may favor the recruitment of endogenous progenitor cells in the area of injury.
Дата добавления: 2015-07-07; просмотров: 1651;
