Water’s Cohesive, Adhesive Properties and Capillary Action
Cohesion allows substances to withstand rupture when placed under stress while adhesion is the attraction between water and other molecules:
· Cohesion holds hydrogen bonds together to create surface tension on water.
· Since water is attracted to other molecules, adhesive forces pull the water toward other molecules.
· Water is transported in plants through both cohesive and adhesive forces; these forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant.
Have you ever filled a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water forms a dome-like shape above the rim of the glass. This water can stay above the glass because of the property of cohesion. In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-gas (water-air) interface, although there is no more room in the glass.
Cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. This is also why water forms droplets when placed on a dry surface rather than being flattened out by gravity. When a small scrap of paper is placed onto the droplet of water, the paper floats on top of the water droplet even though paper is denser (heavier) than the water. Cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. It's even possible to "float" a needle on top of a glass of water if it is placed gently without breaking the surface tension .
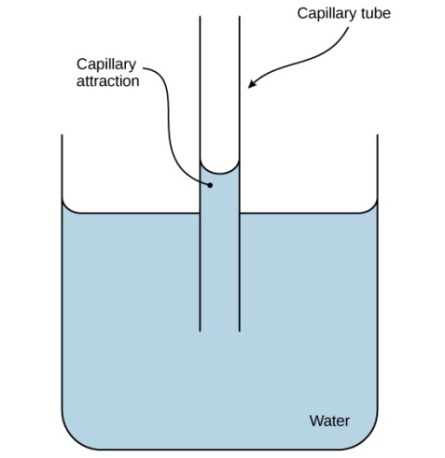
These cohesive forces are related to water's property of adhesion, or the attraction between water molecules and other molecules. This attraction is sometimes stronger than water's cohesive forces, especially when the water is exposed to charged surfaces such as those found on the inside of thin glass tubes known as capillary tubes. Adhesion is observed when water "climbs" up the tube placed in a glass of water: notice that the water appears to be higher on the sides of the tube than in the middle. This is because the water molecules are attracted to the charged glass walls of the capillary more than they are to each other and therefore adhere to it. This type of adhesion is called capillary action .
Adhesion
Capillary action in a glass tube is caused by the adhesive forces exerted by the internal surface of the glass exceeding the cohesive forces between the water molecules themselves.
Why are cohesive and adhesive forces important for life? Cohesive and adhesive forces are important for the transport of water from the roots to the leaves in plants. These forces create a "pull" on the water column. This pull results from the tendency of water molecules being evaporated on the surface of the plant to stay connected to water molecules below them, and so they are pulled along. Plants use this natural phenomenon to help transport water from their roots to their leaves. Without these properties of water, plants would be unable to receive the water and the dissolved minerals they require. In another example, insects such as the water strider use the surface tension of water to stay afloat on the surface layer of water and even mate there.

| 
|
| Surface Tension The weight of the needle is pulling the surface downward; at the same time, the surface tension is pulling it up, suspending it on the surface of the water and keeping it from sinking. Notice the indentation in the water around the needle. | Cohesion & Adhesion Water's cohesive and adhesive properties allow this water strider (Gerris sp.) to stay afloat. |
Even if you've never heard of capillary action, it is still important in your life. Capillary action is important for moving water (and all of the things that are dissolved in it) around. It is defined as the movement of water within the spaces of a porous material due to the forces of adhesion, cohesion, and surface tension.
 Capillary action seen as water climbs to different levels in glass tubes of different diameters.
Capillary action seen as water climbs to different levels in glass tubes of different diameters.
Capillary action occurs because water is sticky, thanks to the forces of cohesion (water molecules like to stay close together) and adhesion (water molecules are attracted and stick to other substances). Adhesion of water to the walls of a vessel will cause an upward force on the liquid at the edges and result in a meniscus which turns upward. The surface tension acts to hold the surface intact. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The height to which capillary action will take water in a uniform circular tube (picture to left) is limited by surface tension and, of course, gravity.
Not only does water tend to stick together in a drop, it sticks to glass, cloth, organic tissues, soil, and, luckily, to the fibers in a paper towel. Dip a paper towel into a glass of water and the water will "climb" onto the paper towel. In fact, it will keep going up the towel until the pull of gravity is too much for it to overcome.
Дата добавления: 2018-11-25; просмотров: 1117;
