Adsorption clearing of gases
Adsorption is the process by which residual molecular forces at the surface of solids attract molecules of gases and vapors. The adsorbing solid is called adsorbent, while the gas which is adsorbed is referred to as the adsorptive. Adsorption of gas molecules occurs at active sites of the solid surface. The active sites are called homogeneous when they all contain the same energy potential. With different energy potentials the sites are said to be heterogeneous. Gas molecules that are adsorbed are called adsorbate. The adsorbed phase consists of a thin gaseous layer, which includes the adsorbate, and a thin solid layer containing the active sites.
During adsorption molecules of gas are besieged on the surface of the firm body just as at condensation, and then are kept on it by physical strengths of an attraction or chemical forces (chemosorption) - depending on the chemical nature of a molecule and a surface. In some systems there can be both kinds of adsorption or intermediate conditions.
The most suitable for adsorption firm substances should possess the following properties: the high porosity, well advanced surface with the big effective area, hardness, propensity to caking at loading in a tower, mechanical durability, simplicity of regeneration after repeated use. These requirements are met by following materials, as coal, alumina, silica gel. They are applied as adsorbents.
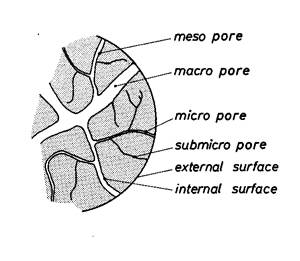
Fig. 2. 29 – Illustration of porous structure of granular adsorbent
| d (nm) = 10-9 m | |
| Macropores Mesopores Micropores Submicropores | 50 ≤ d 2 ≤ d ≤ 50 1 ≤ d ≤ 2 d ≤ 1 |
Adsorbents are divided on three groups:
1) Non-polar firm substances where there is basically a physical adsorption;
2) Polar firm substances where there is a chemical adsorption without change of chemical structure of gas molecules and surface of adsorbent;
3) Surfaces with only chemical adsorption which desorb the gas molecules after chemical reaction – or catalytic reaction when the surface does not undergo changes, or non-catalytic reaction with atoms of adsorbent, and their replacement is required.
Group
Coal - unique non-polar adsorbent having industrial value. The big organic molecules are adsorbed very easy, fine inorganic molecules are adsorbed very bad. The activated coal is received at pyrogenic decomposition of suitable grades of coal or tree.
Group
Polar adsorbents are silica and other siliceous compounds or oxides of metals. Oxides of metals, the activated alumina or bauxite are used for removal of water vapours from gas streams. Siliceous synthetic zeolites (so-called molecular sieves) represent aluminosilicates of sodium or calcium, activated by heating at which crystalization water is removed. The activated synthetic zeolites can be used for drying of gases at high temperatures when silica gel and alumina lose the efficiency. Other field of application of these sorbents is selective adsorption of polar molecules of water, carbon dioxide, ammonia, etc., therefore they are used for clearing of inert and natural gases, for removal СО2 and water from etholene before its polymerization.
Group
There are adsorbing surfaces which enter in chemical reactions with gas molecules, transforming them into more useful substances before desorption. Examples:
1) For removal of sulphurous compounds from coke gas hydrogen sulphide is turned into elementary sulfur at presence iron oxide. Iron oxide is applied as fine dispersed powder, which render on wood shavings or crushed slag (for increasing of surface). Adsorption process for SO2 removal from flue gases is represented on fig. 2.31.
2) Process of chemosorption of SO2 from departing gases on firm adsorbents. Usually in these processes it is used or very cheap raw material (for example, dolomite (the mixture of calcium carbonate and magnesium carbonate) which throw out together with sulfur), or expensive adsorbents from which sulfur is recovered, and adsorbents come back in process.
3) Such compounds as the peroxides, ozone derivatives and other oxygen compounds are easily turned into more simple compounds at the presence of catalyst. The catalyst is the activated coal. The surface of coal is coated with metal copper, silver, platinum and palladium.
4) There are many other examples: oxidation of CO on copper oxide or on I2O5 with formation СО2; bromination of olephins at passing them above the coal, impregnated with bromine; catching of mercury vapours, hydrogen sulphide, fluoric hydrogen on the surface of coal impregnated with various substances (fig. 2.32).
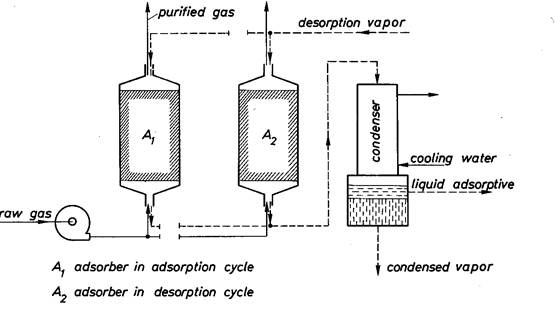
Fig. 2. 30 – Schematic representation of a very simple adsorption unit
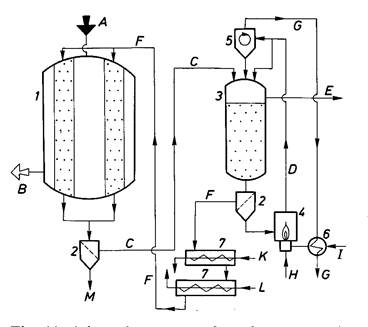
Fig. 2. 31 – Adsorption process for SO2 removal from flue gases: A – raw gas; B – purified gas; C – activated carbon, loaded; D – sand; E – SO2 rich gas; F – activated carbon, regenerated; G – hot gas; H – fuel; I – air; K – cooling water; L – cooling air; M – abrasion; 1 – adsorber; 2 – sieve; 3 – desorber; 4 – sand heater and lift; 5 – cyclone; 6 – head exchanger; 7 – activated carbon transporter
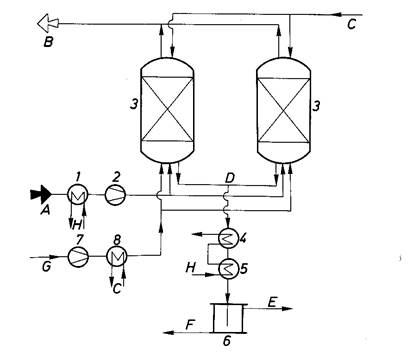
Fig. 2. 32 – Adsorption plant for the removal of organic gases and vapors from waste gases with recovery of solvent: A – raw gas; B – purified gas; C – water vapor; D – desorptive and water vapor; E – solvent, adsorptive; F – condensed water; G – fresh air; H – cooling water; 1 – waste gas cooler; 2 – waste gas ventilator; 3 – adsorber; 4 – condenser; 5 – cooler; 6 – separator; 7 – fresh air ventilator; 8 – fresh air heater
Burning
If polluting substances are easily oxidized, their removal can be carried out by burning of gases. In case of burning of hydrocarbons carbonic gas and water are formed, at burning of sulfides sulphurous gas and water are formed.
There are two types of oxidation processes: the catalytic and the non-catalytic process.
The non-catalytic process, so-called the direct burning or thermal oxidation, is carried out at temperatures 700-8000 С, the catalytic burning - at 250-4000С. Burning is made in the open torch or in the closed chamber.
At burning in torch some hydrocarbons (in particular aromatic compounds with a low ratio carbon - hydrogen) usually give the smoking flame. That it to avoid add water as steam. Thus there is the reaction water steam to hydrocarbons with formation of hydrogen and CO.
In process of catalytic burning the active metal catalyst (platinum or other precious metal, active oxide of metal) on the special carrier is used. The start temperature of catalytic reactions depends on a kind of hydrocarbon in gas. So, hydrogen is oxidized at room temperature, benzene - at 2270 C, methane only in part - at 4040 C.
The most challenge of process of catalytic burning is a gradual deactivation or catalyst poisoning at long service life or at unexpected occurrence of poisons in a gas stream. Even traces of a certain type of pollutant may poison the catalyst.
Leaving the chamber gases are passed through heater or are thrown out in an atmosphere.
The advantage of the thermal oxidation process is the nearly complete removal of the pollutant without producing any new pollutants or other environmental problems.
Дата добавления: 2017-11-04; просмотров: 1487;
