III. The Rocky Shores 3 страница
As the tide falls the tips of the weeds, lacking support, float out horizontally across the surface. Then the cloud shadows darken and a deepening gloom settles over the floor of the forest. As the overlying layer of water thins and gradually drains away, the weeds, still stirring, still responsive to each pulsation of the tide, drift closer to the rock floor and finally lie prostrate upon it, all their life and movement in abeyance.
By day an interval of quiet settles over the jungles of the land, when the hunters lie in their dens, and the weak and the slow hide from the daylight; so on the shore a waiting lull comes with every ebbing of the tide.
The barnacles furl their nets and swing shut the twin doors that exclude the drying air and hold within the moisture of the sea. The mussels and the clams withdraw their feeding tubes or siphons and close their shells. Here and there a starfish, having invaded the forest from below on the previous high tide and incautiously lingered, still clasps a mussel within its sinuous arms, gripping the shells with the sucker‑tipped ends of scores of slender tube feet. Pushing under and among the horizontal fronds of the weed, as a man would make his way with difficulty through trees blown down by a storm, a few crabs are active, digging their little slanting pits to expose the clams buried in the mud. Then they crack away pieces of shell with their heavy claws, while they hold the clam in the tips of the walking legs.
A few hunters and scavengers come down from the upper tidelands. The little gray‑cloaked tide‑pool insect, Anurida, wanders down from the upper shore and scurries over the rock floor, hunting out mussels with gaping shells, or dead fish, or fragments of crabs left by gulls. Crows walk about over the weeds; they sort them over strand by strand until they find a periwinkle hidden in the weed, or clinging to a rock that lies under the sodden cloak of the algae. Then the crow holds the shell in the strong toes of one foot, while with its beak it deftly extracts the snail.
The pulse of the returning tide at first beats gently. The advance during the beginning of the six‑hour rise to high‑water mark is slow, so that in two hours only a quarter of the intertidal zone has been covered. Then the pace of the water quickens. For the next two hours the tidal currents are stronger and the rising waters advance twice as far as in the first period; then again the tide slackens its pace for a leisurely advance over the upper shore. The rockweeds, covering the middle band of shore, receive the shock of heavier waves than the relatively bare shore above, yet their cushioning effect is so great that the animals that cling to them or live on the rock floor below them are far less affected by the surf than those of the upper rocks, or those of the zone below which experience all the heavy drag from the backwash of waves that break as the tide is advancing rapidly over the middle shore.
Darkness brings the jungles of the land to life, but the night of the rockweed jungles is the time of the rising tide, when water pours in under the masses of weed, stirring out of their low‑tide quiescence all the inhabitants of this forest.
As the water from the open sea floods the floor of the weed jungles, shadows flicker again above the ivory cones of the barnacles as the almost invisible nets reach out to gather what the tide has brought. The shells of clams and mussels again open slightly and little vortices of water are drawn down, funneling into the complex straining mechanisms within the shellfish all the little spheres of marine vegetables that are their food.
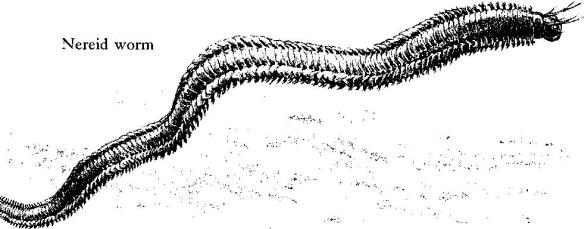
Nereid worms emerge from the mud and swim off to other hunting grounds; if they are to reach them they must elude the fishes that have come in with the tide, for on the flood tide the rockweed forests become one with the sea and with its hungry predators.
Shrimp flicker in and out through the open spaces of the forest; they seek small crustaceans, baby fish, or minute bristle worms, but in their turn are pursued by following fish. Starfish move up from the great meadows of sea moss lower on the shore, hunting the mussels that grow on the floor of the forest.
The crows and the gulls are driven out of the tidelands. The little gray, velvet‑cloaked insects move up the shore or, finding a secure crevice, wrap themselves in a glistening blanket of air to wait for the falling of the tide.
The rockweeds that create this intertidal forest are descendants of some of the earth’s most ancient plants. Along with the great kelps lower on the shore, they belong to the group of brown seaweeds, in which the chlorophyll is masked by other pigments. The Greek name for the brown algae–the Phaeophyceae– means “the dusky or shadowy plants.” According to some theories, they arose in that early period when the earth was still enveloped in heavy clouds and illuminated only by feeble rays of sunlight. Even today the brown seaweeds are plants of dim and shadowed places–the deep submarine slopes where giant kelps form dusky jungles and the dark rock ledges from which the oarweeds send their long ribbons streaming into the tides. And the rockweeds that grow between the tide lines do so on northern coasts, visited often by cloud and fog. Their rare invasions of the sunny tropics are accomplished under a protective cover of deep water.
The brown algae may have been the first of the sea plants to colonize the shore. They learned to adjust themselves to alternating periods of submersion and exposure on ancient coastlines swept by strong tides; they came as close to a land existence as they could without actually leaving the tidal zone.
One of the modern rockweeds, the channeled wrack of European shores, lives at the extreme upper edge of the tidelands. In some places its only contact with the sea is an occasional drenching with spray. In sun and air its fronds become blackened and crisp so that one would think it had surely been killed, but with the return of the sea its normal color and texture are restored.
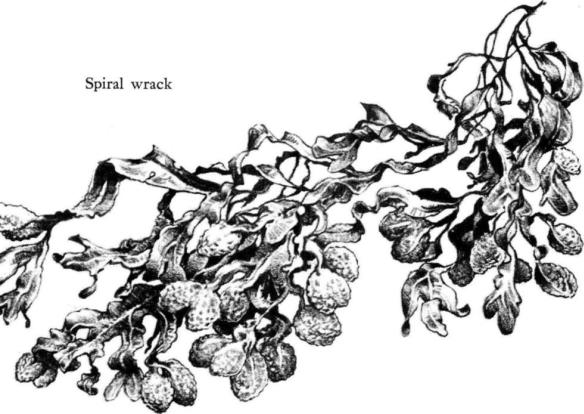
The channeled wrack does not grow on the American Atlantic coast, but there a related plant, the spiral wrack, comes almost as far out of the sea. It is a weed of low growth, whose short sturdy fronds end in turgid, rough‑textured swellings. Its heaviest growth is above the high‑water mark of the neap tides, so of all the rockweeds it lives closest inshore or nearest the water line of exposed ledges. Although it spends nearly three‑fourths of its life out of water, it is a true seaweed and its splashes of orange‑brown color on the upper shore are a symbol of the threshold of the sea.
These plants, however, are but the outlying fringe of the intertidal forest, which is an almost pure stand of two other rockweeds–the knotted wrack and the bladder wrack. Both are sensitive indicators of the force of the surf. The knotted wrack can live in profusion only on shores protected from heavy waves, and in such places is the dominant weed. Back from the headlands, on the shores of bays and tidal rivers where surf and tidal surge are subdued by remoteness from the open sea, the knotted wrack may grow taller than the tallest man, though its fronds are slender as straws. The long surge of the swell in sheltered water places no great strain on its elastic strands. Swellings or vesicles on the main stems or fronds contain oxygen and other gases secreted by the plant; these act as buoys when the weeds are covered by the tide. The bladder wrack has greater tensile strength and so can endure the sharp tugging and pulling of moderately heavy surf. Although it is much shorter than the knotted wrack it also needs the help of air bladders to rise in the water. In this species the bladders are paired, one of each pair on either side of the strong midrib; the bladders, however, may fail to develop where the plants are subjected to much pounding by surf, or when they grow at the lower levels of the tidal zone. At some seasons the ends of the branches of this wrack swell into bulbous, almost heart‑shaped structures; from these the reproductive cells are liberated.
The sea wracks have no roots, but instead grip the rocks by means of a flattened, disc‑like expansion of their tissues. It is almost as though the base of each weed melted a little, spreading over the rock and then congealing, thereby creating a union so firm that only the thundering seas of a very heavy storm, or the grinding of shore ice, can tear away the plants. The seaweeds do not have a land plant’s need of roots to extract minerals from the soil, for they are bathed almost continuously by the sea and so live within a solution of all the minerals they need for life. Nor do they need the rigid supporting stem or trunk by which a land plant reaches upward into sunlight–they have only to yield themselves to the water. And so their structure is simple–merely a branching frond arising from the holdfast, with no division into roots and stems and leaves.
Looking at the prostrate, low‑tide forests of the rockweeds that cover the shore with a many‑layered blanket, one would suppose that the plants must spring from every available inch of rock surface. But actually the forest, when it rises and comes to life with the flooding tide, is fairly open and sprinkled with clearings. On my own shore in Maine, where the tides rise and fall over a wide expanse of intertidal rock, and the knotted wrack spreads its dark blanket between the high and low waters of the neap tides, the areas of open rock around the holdfast of each plant are sometimes as much as a foot in diameter. From the middle of such a clearing the plant rises, its fronds dividing repeatedly, until the upper branchings extend out over an area several feet across.
Far below, at the base of the fronds that swing with the undulation of the passing waves, the rocks are stained with vivid hues, painted in crimson and emerald by the activities of sea plants so minute that even in their thousands they seem but part of the rock, a surface revelation of jewel tones within. The green patches are growths of one of the green algae. The individual plants are so small that only a strong lens could reveal their identity–lost, as individual blades of grass are lost in the lush expanse of a meadow, in the spreading verdant stain created by the mass. Amid the green are other patches of a rich and intensely glowing red, and again the growth is not separable from the mineral floor. It is a creation of one of the red seaweeds, a form that secretes lime in thin and closely adhering crusts over the rocks.
Against this background of glowing color the barnacles stand out with sharp distinctness, and in the clear water that pours through the forest like liquid glass, their cirri flicker in and out‑extending, grasping, withdrawing, taking from the inpouring tides those minute atoms of life that our eyes cannot see. Around the bases of small wave‑rounded boulders the mussels lie as though at anchor, held by gleaming lines spun by their own tissues. Their paired blue shells stand a little apart, the space between them revealing pale brown tissues with fluted edges.
Some parts of the forest are less open. In these the clumps of rockweeds rise from a short turf or undergrowth consisting chiefly of the flat fronds of Irish moss, with sometimes dark mats of another plant with the texture of Turkish toweling. And like a tropical jungle with its orchids, this sea forest has the counterpart of airplants in the epiphytic tufts of a red seaweed that grows on the fronds of the knotted wrack. Polysiphonia seems to have lost–or perhaps it never had–the ability to attach directly to the rocks and so its dark red balls of finely divided fronds cling to the wracks, and by them are lifted up into the water.
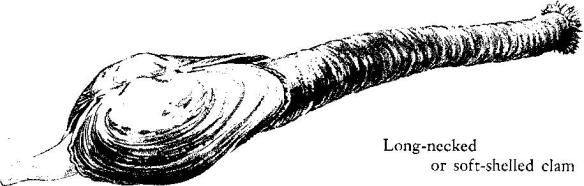
In the areas between the rocks and under loose boulders a substance that is neither sand nor mud has accumulated. It consists of minute and water‑ground bits of the remains of sea creatures–the shells of mollusks, the spines of sea urchins, the opercula of snails. Clams live in pockets of this soft substance, digging down until they are buried to the tips of their siphons. Around the clams the mud is alive with ribbon worms, thin as threads, scarlet of color, each a small hunter searching out minute bristle worms and other prey. Here also are the nereids, given the Latin name for sea nymph because of their grace and iridescent beauty. The nereids are active predators that leave their burrows at night to search for small worms, crustaceans, and other prey. In the dark of the moon certain species gather at the surface in immense spawning swarms. Curious legends have become associated with them. In New England the so‑called clam worm, Nereis virens, often takes shelter in empty clam shells. Fishermen, accustomed to finding it thus, believe it is the male clam.
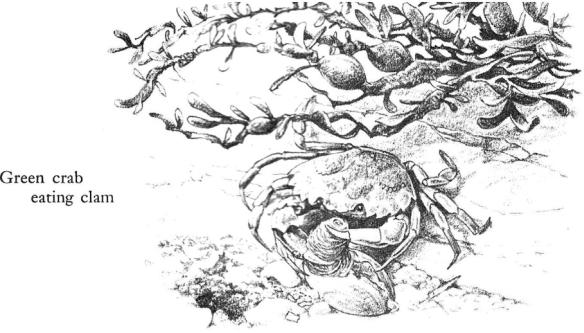
Crabs of thumbnail size live in the weed and come down to hunt in these areas. They are the young of the green crab; the adults live below the tide lines on this shore except when they come into the shelter of the weeds to molt. The young crabs search the mud pockets, digging out pits and probing for clams that are about their own size.
Clams, crabs, and worms are part of a community of animals whose lives are closely interrelated. The crabs and the worms are the active predators, the beasts of prey. The clams, the mussels, and the barnacles are the plankton feeders, able to live sedentary lives because their food is brought to them by each tide. By an immutable law of nature, the plankton feeders as a group are more numerous than those that prey on them. Besides the clams and other large species, the rockweeds shelter thousands of small beings, all of them busy with filtering devices of varying design, straining out the plankton of each tide. There is, for example, a small, plumed worm called Spirorbis. Seeing it for the first time, one would certainly say that it is no worm, but a snail, for it is a tube‑builder, having learned some feat of chemistry that allows it to secrete about itself a calcareous shell or tube. The tube is not much larger than the head of a pin and is wound in a flat, closely coiled spiral of chalky whiteness, its form strongly suggesting some of the land snails. The worm lives permanently within the tube, which is cemented to weed or rock, thrusting out its head from time to time to filter food animals through the fine filaments of its crown of tentacles. These exquisitely delicate and filmy tentacles serve not only as snares to entangle food but as gills for breathing. Among them is a structure like a long‑stemmed goblet; when the worm draws back into its tube the goblet or operculum closes the opening like a neatly fitted trap door.
The fact that the tube worms have managed to live in the intertidal zone for perhaps millions of years is evidence of a sensitive adjustment of their way of life, on the one hand to conditions within the surrounding world of the rockweeds, on the other to vast tidal rhythms linked with the movements of earth, moon, and sun.
In the inmost coils of the tube are little chains of beads wrapped in cellophane–or so they appear. There are about twenty beads in a chain. The beads are developing eggs. When the embryos have developed into larvae, the cellophane membranes rupture and the young are sent forth into the sea. By keeping the embryonic stages within the parental tube Spirorbis protects its young from enemies and assures that the infant worms will be in the intertidal zone when they are ready to settle. Their period of active swimming is short–at most an hour or so, and well contained within a single rising or falling of the tide. They are stout little creatures with bright red eye spots; perhaps the larval eyes help in locating a place for attachment but in any event they degenerate soon after the larva settles.
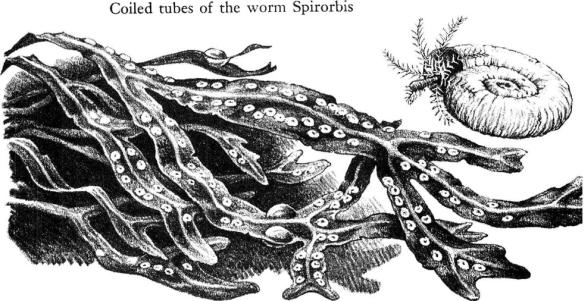
In the laboratory, under my microscope, I have watched the larvae swimming about busily, all their little bristles whirring, then sometimes descending to the glass floor of their dish to bump it with their heads. Why and how do the infant tube worms settle in the same sort of place their ancestors chose? Apparently they make many trials, reacting more favorably to smooth surfaces than to rough, and displaying a strong instinct of gregariousness that leads them to settle by preference where others of their kind are already established. These tendencies help to keep the tube worms within their comparatively restricted world. There is also a response, not to familiar surroundings, but to cosmic forces. Every fortnight, on the moon’s quarter, a batch of eggs is fertilized and taken into the brood chamber to begin its development. And at the same time the larvae that have been made ready during the previous fortnight are expelled into the sea. By this timing–this precise synchronizing with the phases of the moon–the release of the young always occurs on a neap tide, when neither the rise nor the fall of the water is of great extent, and even for so small a creature the chances of remaining within the rockweed zone are good.
Sea snails of the periwinkle tribe inhabit the upper branches of the weeds at high tide or take shelter under them when the tide is out. The orange and yellow and olive‑green colors of their smoothly rounded, flat‑topped shells suggest the fruiting bodies of the rockweeds, and perhaps the resemblance is protective. The smooth periwinkle, unlike the rough, is still an animal of the sea; the salty dampness it requires is provided by the wet and dripping fronds of the seaweeds when the tide is out. It lives by scraping off the cortical cells of the algae, seldom if ever descending to the rocks to feed on the surface film as related species do. Even in its spawning habits the smooth periwinkle is a creature of the rockweeds. There is no shedding of eggs into the sea, no period of juvenile drifting in the currents. All the stages of its life are lived in the rockweeds–it knows no other home.
Curious about the early stages of this abundant snail, I have gone down into my own rockweed forests on the summer low tides to search for them. Sorting over the prostrate wrack, examining its long strands for some signs of what I sought, I have occasionally been rewarded by discovering transparent masses of a substance like tough jelly, tightly adhering to the fronds. They averaged perhaps a quarter‑inch long and half as wide. Within each mass 1 could see the eggs, round as bubbles, dozens of them embedded in the confining matrix. One such egg mass that I carried to the microscope contained a developing embryo within the membranes of each egg. They were clearly molluscan, but so undifferentiated that I could not have said what mollusk lay nascent within. In the cold waters of its home, about a month would intervene from the egg to the hatching stage, but in the warmer temperatures of the laboratory the remaining days of development were reduced to hours. The following day each sphere contained a tiny baby periwinkle, its shell completely formed, apparently ready to emerge and take up its life on the rocks. How do they hold their places there, I wondered, as the weeds sway in the tides and occasional storms send waves pounding in over the shore? Later in the summer there was at least a partial answer. I noticed that many of the air vesicles of the wracks bore little perforations, as though they had been chewed or punctured by some animal. I slit some of these vesicles carefully so that I might look inside. There, secure in a green‑walled chamber, were the babies of the smooth periwinkle–from two to half a dozen of them sharing the refuge of a single vesicle, secure alike against storms and enemies.

Down near the low water of the neap tides the hydroid Clava spreads its velvet patches on the fronds of the knotted wrack and the bladder wrack. Rising from its point of attachment like a plant from its root clump, each cluster of tubular animals looks like nothing so much as a spray of delicate flowers, shading from pink to rose and fringed with petal‑like tentacles, all nodding in the water currents as woodland flowers nod in a gentle wind. But the swaying movements are purposive ones by which the hydroid reaches into the currents for food. In its way it is a voracious little jungle beast, all its tentacles studded with batteries of stinging cells that can be shot into its victims like poisoned arrows. When, in their ceaseless movements, the tentacles come into contact with a small crustacean or worm or the larva of some sea creature, a shower of darts is released; the prey animal becomes paralyzed and is seized and conveyed to the mouth by the tentacles.
Each of these colonies now established on the wracks came from a little swimming larva that once settled there, shed the hairy cilia by which it swam, attached itself, and began to elongate into a little plantlike being. A crown of tentacles formed at its free end. In time, from the base of the tubular creature, a seeming root, or stolon, began to creep over the rockweed, budding off new tubes, each complete with mouth and tentacles. So all the numerous individuals of the colony originated in a single fertilized ovum that yielded the wandering larva.
In season, the plantlike hydroid must reproduce, but by a strange circumstance it cannot itself yield the germ cells that would give rise to new larvae, for it can reproduce only non‑sexually, by budding. So there is a curious alternation of generations, found again and again in many members of the large coelenterate group to which the hydroids belong, by which no individual produces offspring that resemble itself, but each is like the grandparental generation. Just below the tentacles of an individual Clava the buds of the new generation are produced–the alternate generation that intervenes between colonies of hydroids. They are pendent clusters shaped like berries. In some species the berries, or medusa buds, would drop from the parent and swim away–tiny, bell‑shaped things like minute jellyfish. Clava, however, does not release its medusae but keeps them attached. Pink buds are male medusae; purple ones are female. When they are mature, each sheds its eggs or sperm into the sea. When fertilized, the eggs begin to divide and through their development yield the little protoplasmic threads of larvae, which swim off through unknown waters to found some distant colonies.
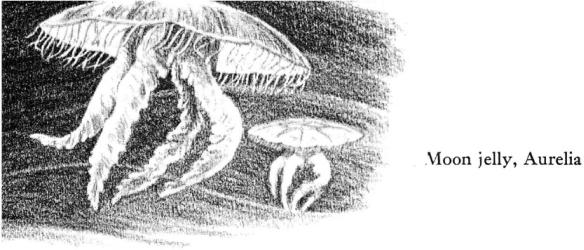
During many days of midsummer, the incoming tides bring the round opalescent forms of the moon jellies. Most of these are in the weakened condition that accompanies the fulfillment of their life cycle; their tissues are easily torn by the slightest turbulence of water, and when the tide carries them in over the rockweeds and then withdraws, leaving them there like crumpled cellophane, they seldom survive the tidal interval.
Each year they come, sometimes only a few at a time, sometimes in immense numbers. Drifting shoreward, their silent approach is unheralded even by the cries of sea birds, who have no interest in the jellyfish as food, for their tissues are largely water.

During much of the summer they have been drifting offshore, white gleams in the water, sometimes assembling in hundreds along the line of meeting of two currents, where they trace winding lines in the sea along these otherwise invisible boundaries. But toward autumn, nearing the end of life, the moon jellies offer no resistance to the tidal currents, and almost every flood tide brings them in to the shore. At this season the adults are carrying the developing larvae, holding them in the flaps of tissue that hang from the under surface of the disc. The young are little pear‑shaped creatures; when finally they are shaken loose from the parent (or freed by the stranding of the parent on the shore), they swim about in the shallow water, sometimes swarms of them together. Finally they seek bottom and each becomes attached by the end that was foremost when it swam. As a tiny plantlike growth, about an eighth of an inch high and bearing long tentacles, this strange child of the delicate moon jelly survives the winter storms. Then constrictions begin to encircle its body, so that it comes to resemble a pile of saucers. In the spring these “saucers” free themselves one after another and swim away, each a tiny jellyfish, fulfilling the alternation of the generations. North of Cape Cod these young grow to their full diameter of six to ten inches by July; they mature and produce eggs and sperm cells by late July or August; and in August and September they begin to yield the larvae that will become the attached generation. By the end of October all of the season’s jellyfish have been destroyed by storms, but their offspring survive, attached to the rocks near the low‑tide line or on nearby bottoms offshore.
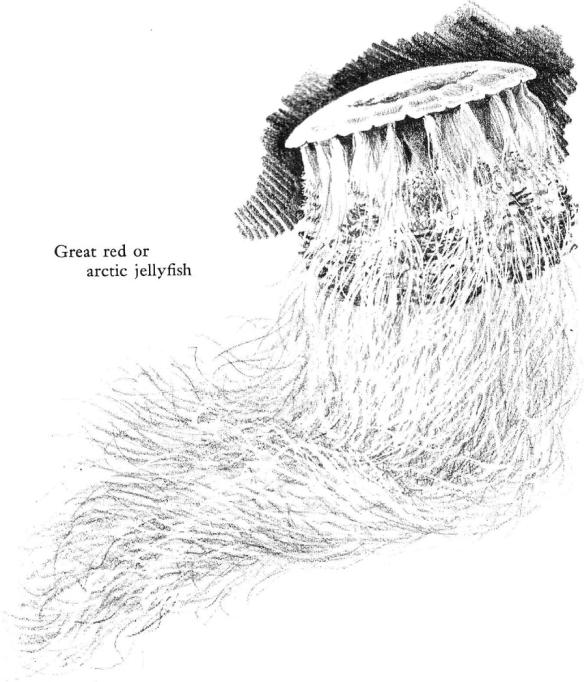
If the moon jellies are symbols of the coastal waters, seldom straying more than a few miles offshore, it is otherwise with the great red jellyfish, Cyanea, which in its periodic invasions of bays and harbors links the shallow green waters with the bright distances of the open sea. On fishing banks a hundred or more miles offshore one may see its immense bulk drifting at the surface as it swims lazily, its tentacles sometimes trailing for fifty feet or more. These tentacles spell danger for almost all sea creatures in their path and even for human beings, so powerful is the sting. Yet young cod, haddock, and sometimes other fishes adopt the great jellyfish as a “nurse,” traveling through the shelterless sea under the protection of this large creature and somehow unharmed by the nettle‑like stings of the tentacles.
Like Aurelia, the red jellyfish is an animal only of the summer seas, for whom the autumnal storms bring the end of life. Its offspring are the winter plantlike generation, duplicating in almost every detail the life history of the moon jelly. On bottoms no more than two hundred feet deep (and usually much less), little half‑inch wisps of living tissue represent the heritage of the immense red jellyfish. They can survive the cold and the storms that the larger summer generation cannot endure; when the warmth of spring begins to dissipate the icy cold of the winter sea they will bud off the tiny discs that, by some inexplicable magic of development, grow in a single season into the adult jellyfish.
As the tide falls below the rockweeds, the surf of the sea’s edge washes over the cities of the mussels. Here, within these lower reaches of the intertidal zone, the blue‑black shells form a living blanket over the rocks. The cover is so dense, so uniform in its texture and composition, that often one scarcely realizes that this is not rock, but living animals. In one place the shells, unimaginable in number, are no more than a quarter of an inch long; in another the mussels may be several times as large. But always they are packed so closely together, neighbor against neighbor, that it is hard to see how any one of them can open its shells enough to receive the currents of water that bring its food. Every inch, every hundredth of an inch of space, has been taken over by a living creature whose survival depends on gaining a foothold on this rocky shore.
The presence of each individual mussel in this crowded assemblage is evidence of the achievement of its unconscious, juvenile purpose, an expression of the will‑to‑live embodied in a minute transparent larva once set adrift in the sea to find its own solid bit of earth for attachment, or to die.
The setting adrift takes place on an astronomical scale. Along the American Atlantic coast the spawning season of the mussels is protracted, extending from April into September. What induces a wave of spawning at any particular time is unknown, but it seems clear that the spawning of a few mussels releases chemical substances into the water, and that these react on all mature individuals in the area and set them to pouring their eggs and milt into the sea. The female mussels discharge the eggs in a continuing, almost endless stream of short little rod‑like masses–hundreds, thousands, millions of cells, each potentially an adult mussel. One large female may release up to twenty‑five million at a single spawning. In quiet water the eggs drift gently to the bottom, but in the normal conditions of surf or swiftly moving currents they are at once possessed by the sea and carried away.
Simultaneously with the outflow of eggs, the water has become cloudy with the milt poured into the water by the male mussels, the number of individual sperm cells defying all attempts at calculation. Dozens of them cluster about a single egg, pressing against it, seeking entrance. But one male cell, and one only, is successful. With the entrance of this first sperm cell, an instantaneous physical change takes place in the outer membranes of the egg, and from this moment it cannot again be penetrated by a spermatozoan.
Дата добавления: 2015-05-13; просмотров: 1203;
