Гидраты индивидуальных углеводородных и неуглеводородных компонентов.
Диаграммы давление – температура систем CH4 + H2O (или N2 + H2O)
Гидрат метана впервые был получен в 1888 г.
Рассмотрим диаграммы давление – температура для систем CH4 + H2O или N2 + H2O схематически показанные на рисунке при условиях, охватывающих гидратную область.
Так как метан – основной компонент природного газа, эта диаграмма, а также связанная с ней T–x диаграмма обеспечивают понимание всей гидратной области газовых систем, не содержащих жидкую углеводородную фазу.
На диаграммах используются следующие символы:
I – лёд, LW – жидкая вода, H - гидрат, V - пар, и LHC – жидкий углеводород, перечисленные в порядке снижения содержания воды.
Согласно правилу фаз Гиббса, двухкомпонентная система, такая как метан + вода представляется на диаграмме давление – температура как область (для двух фаз), и линией (три фазы) или точкой (четыре фазы).
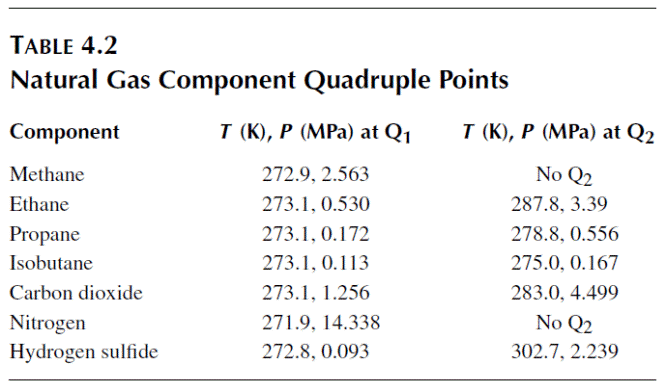
Рассмотрим квадрупольную точку (Q1), в которой четыре фазы (I–LW–H–V) сосуществуют. Температура квадрупольной точки приблизительно лежит в окрестности 273,16 K для всех гидратов, однако давления для разных компонентов разнятся более значительно (например, 0.0113 МПа для i-C4H10, 2.56 МПа для CH4, and 14.3 МПа for N2). Квадрупольная точка (Q1) является стартовой точкой для линий равновесного сосуществования четырёх и трёх фаз:
1. The LW–H–V line has pressure–temperature conditions of the most interest in natural gas systems.
2. The I–H–V line, which has a lower P–T slope than the LW–H–V line. Note that there is a data paucity in the region below 273 K, which is avoided industrially (hence a lack of funding) due to problems with ice formation.
3. The I–LW–H line rises vertically from the quadruple point, with very large pressure changes for small temperature changes, as typified by incompressible phases.
4. The I–LW–V line that connects the quadruple point to the water triple point (I–LW–VW) (273.16 K, 0.62 kPa), denotes the transition between water and ice without hydrate formation. Since Q1 approximates 273 K for all natural gas systems, the I–LW–V line extends almost vertically below Q1 to 0.62 kPa.
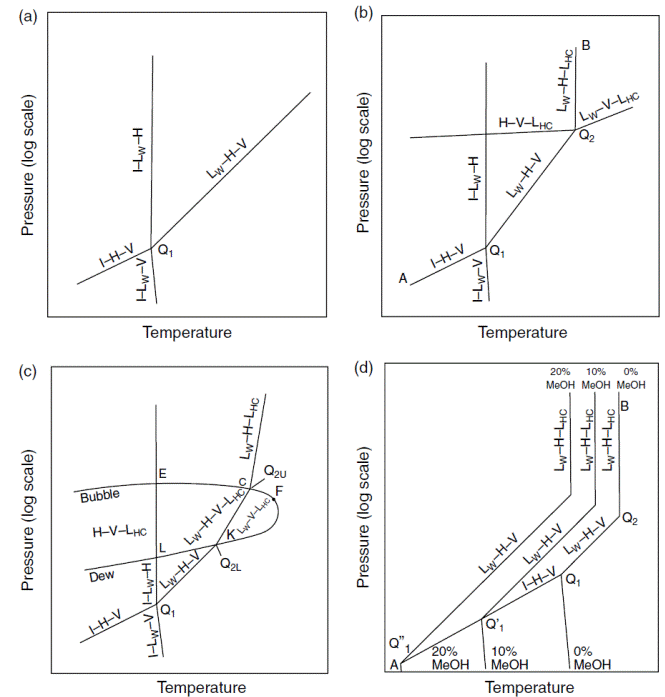
Pressure–temperature diagrams. (a) Methane + water or nitrogen + water system in the hydrate region. (b) Hydrocarbon + water systems with upper quadruple points. (c) Multicomponent natural gas+water systems. (d) Hydrocarbon+water systems with upper quadruple points and inhibitors.
In all of Figure 4.2 note that composition, a third dimension, has been compressed onto the two shown (pressure and temperature), so that the lines shown may project from or into the page.
The equation of Table 4.1 from Kamath (1984) enables prediction of the most common regions of interest of simple natural gas components—the pressure and temperature conditions for both LW–H–V and I–H–V. When using the equation, carefully note the temperature limits. It would be a mistake (for example) to extend the prediction of the LW–H–V region beyond the temperature of either quadruple point Q1 or Q2 (given in Table 4.2), where those three phases cannot exist.
1. The pressures and temperatures of the LW–H–V and the I–H–V lines mark the limits to hydrate formation. At higher temperatures or lower pressures of both lines, hydrate cannot form and the system will contain only aqueous and hydrocarbon fluid phases, while hydrate formation can occur to the left of LW–H–V and I–H–V. Since ice and hydrates both cause flow problems, a gas pipeline rule of thumb is to keep the system temperature above the ice point and to the right of the LW–H–V and the I–H–V lines, or to displace the LW–H–V line below the pipeline operating conditions by injection of a thermodynamic inhibitor such as methanol.
2. The LW–H–V line has no upper pressure or temperature limit because the pure methane (or nitrogen) vapor–liquid critical points (at 191 and 126 K respectively) are far below the quadruple point Q1. Such low critical temperatures prevent intersection of the vapor pressure line with the LW–H–V line above 273 K to produce an upper quadruple point.
3. Similarly, no upper pressure limit to the I–LW–H line has been found. Note that these phases are all incompressible, so that a very large pressure change results from only a small temperature change, in a closed system.
4. The areas between the three-phase lines represent the two-phase region held in common with the bounding three-phase lines. For instance, the area between LW–H–V and I–H–V is the H–V region in which hydrates are in equilibrium only with vapor (water saturated). Similarly, the LW–H two-phase region exists between LW–H–V and I–LW–H lines, and the I–H two-phase region exists between the I–LW–H and I–H–V lines. In this two-dimensional plot the two-phase regions overlap, indicating that the three-phase lines are not all in the plane of the page, but have been compressed into two dimensions, from three, with the third dimension being composition. The compression of the composition axis onto the P–T plane causes the two-phase regions to overlap. Two-phase regions are discussed with T–x diagrams in Section 4.1.5.
5. The diagram schematic is the same for simple hydrate systems of sI (CH4 + H2O) and sII (N2 + H2O) as well as those of fixed natural gas mixture compositions, without a liquid hydrocarbon phase. Systems containing a liquid hydrocarbon are similar in behavior to the
C3H8 + H2O diagram, discussed in Section 4.1.2.
It has been shown (Bansal et al., 1993) that curvature in the LW–H–V line in
Figure 4.2a results if the guest vapor–liquid critical temperature is slightly below
the LW–H–V conditions.
4.1.2 Systems (e.g., H2O + C2H6, C3H8, or i-C4H10) with Upper Quadruple Points
Figure 4.2b shows the equivalent of Figure 4.2a to be slightly more complex for systems such as ethane+water, propane+water, isobutane+water, or water with the two common noncombustibles, carbon dioxide or hydrogen sulfide. These systems have a three-phase (LW–V–LHC) line at the upper right in the diagram.
This line is very similar to the vapor pressure (V–LHC) line of the pure hydrocarbon,
because the presence of the almost pure water phase adds a very low vapor pressure
(a few mmHg at ambient conditions) to the system.
Figure 4.2b shows that at the intersection of the LW–V–LHC line with the
LW–H–V line, a second quadruple point (Q2 = LW–H–V–LHC) is formed. Measured
upper quadruple points for simple natural gas components are shown in
Table 4.2. Point Q2 is the origin for two additional three-phase lines: (1) a LW–
H–LHC line that is almost vertical due to the three incompressible phases and
(2) a H–V–LHC line, of less concern, because it exists within the LW–H–LHC and
the LW–H–V boundaries.
For systems with two quadruple points, the hydrate region is bounded by line
I–H–V at conditions below Q1, line LW–H–V between Q1 and Q2, as well as line
LW–H–LHC at conditions above Q2. Hydrates can form at lower temperatures and
higher pressures to the left of the region enclosed by the three lines in Figure 4.2b; to
the right, no hydrates are possible. Upper quadruple pointQ2 is often approximated
as the maximum temperature of hydrate formation, because line LW–H–LHC is
almost vertical; however see data in Chapter 6 for exceptions.
In Figure 4.2b, the areas between the three-phase lines represent two-phase
regions held in common with the three-phase lines. The area bound by three
three-phase lines (I–LW–H, LW–H–V, and LW–H–LHC) is the LW–H region in
which hydrates are in equilibrium only with liquid water. Similarly, the H–V
region is between the three three-phase lines (H–V–LHC, LW–H–V, and I–H–V).
Finally, the H–LHC two-phase region exists between LW–H–LHC and H–V–LHC
lines and the I–H two-phase region exists between the I–LW–H and I–H–V lines.
Estimation Techniques for Phase Equilibria 201
See Section 4.1.5 for a T–x diagram with another perspective of these two-phase
regions.
Note that the last paragraph contains two-phase regions(H–V,H–LHC, and I–H)
for hydrate equilibrium with a phase that is not liquid water. There is a common
misconception that hydrates cannot form without a liquid water phase, a condition
clearly possible in these diagrams. Professor Kobayashi’s laboratory measured
hydrate conditions without a free water phase from vapor or liquid systems from
1973 to 2000. Such equilibria are of interest for gas and gas condensate pipelines
without a free water phase.
Дата добавления: 2014-12-09; просмотров: 1750;
