The Potentiometer and the pH Meter
There are two commonly used for making potentiometric measurements.
The potentiometer is a device which is normally used for the measurement of potentials in low resistance circuits and as a result is only rarely applied.
The pH Meter, which is a voltmeter, is a voltage measuring device designed for use with high resistance glass electrodes and can be used with both low and high resistance circuits. During a measurement the voltage is converted to a current amplification via an ac circuit and these are therefore high input impedance devices. For convenience when making pH measurements, the voltage reading can be converted directly to pH units.
The pH meter measures the pH of a solution using an ion-selective electrode (ISE) that responds to the H+ concentration of the solution. The pH electrode produces a voltage that is proportional to the concentration of the H+ concentration, and making measurements with a pH meter is therefore a form of potentiometry. The pH electrode is attached to control electronics which convert the voltage to a pH reading and displays it on a meter.
A pH meter consists of a H+-selective membrane, an internal reference electrode, an external reference electrode, and a meter with control electronics and display. Commercial pH electrodes usually combine all electrodes into one unit that are then attached to the pH meter.
Picture of a pH meter

Ion-Selective Electrodes (ISE)
An Ion-Selective Electrode (ISE) produces a potential that is proportional to the concentration of an analyte. Making measurements with an ISE is therefore a form of potentiometry. The most common ISE is the pH electrode, which contains a thin glass membrane that responds to the H+ concentration in a solution.
The potential difference across an ion-sensitive membrane is:
E = K - (2.303RT/nF)log(a)
where K is a constant to account for all other potentials, R is the gas constant, T is temperature, n is the number of electrons transferred, F is Faraday's constant, and a is the activity of the analyte ion. A plot of measured potential versus log(a) will therefore give a straight line.
ISEs are susceptible to several interferences. Samples and standards are therefore diluted 1:1 with total ionic strength adjuster and buffer (TISAB). The TISAB consists of 1 M NaCl to adjust the ionic strength, acetic acid/acetate buffer to control pH, and a metal complexing agent.
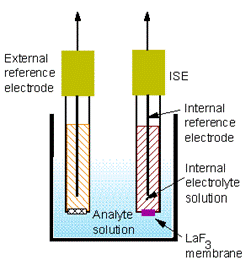
ISEs consist of the ion-selective membrane, an internal reference electrode, an external reference electrode, and a voltmeter.
Commercial ISEs often combine the two electrodes into one unit that are then attached to a pH meter.
Picture of a commercial fluoride ISE

Schematic of an ISE measurement
Дата добавления: 2018-11-25; просмотров: 2011;
