Water’s States: Gas, Liquid, and Solid
Water Properties and Hardness
Water is a transparent fluid which forms the world's streams, lakes, oceans and rain, and is the major constituent of the fluids of living things.
Water (H2O) is the most abundant compound on Earth's surface, covering 70 percent of the planet. In nature, water exists in liquid, solid, and gaseous states. It is in dynamic equilibrium between the liquid and gas states atstandard temperature and pressure. At room temperature, it is a tasteless and odorless liquid, nearly colorless with a hint of blue. Many substances dissolve in water and it is commonly referred to as the universal solvent. Because of this, water in nature and in use is rarely pure and some properties may vary from those of the pure substance. However, there are also many compounds that are essentially, if not completely, insoluble in water. Water is the only common substance found naturally in all three common states of matter and it is essential for all life on Earth. Water makes up 55% to 78% of the human body.
Water’s States: Gas, Liquid, and Solid
The orientation of hydrogen bonds as water changes states dictates the properties of water in its gaseous, liquid, and solid forms.
· As water is boiled, kinetic energy causes the hydrogen bonds to break completely and allows water molecules to escape into the air as gas (steam or water vapor).
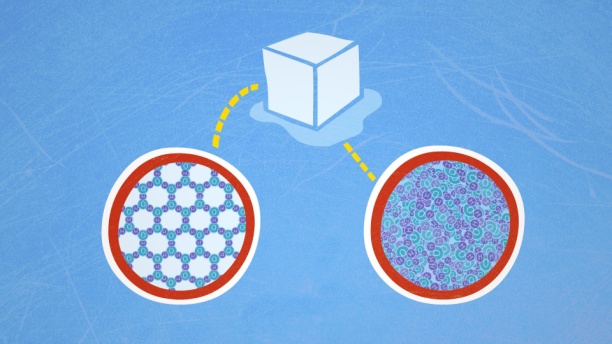
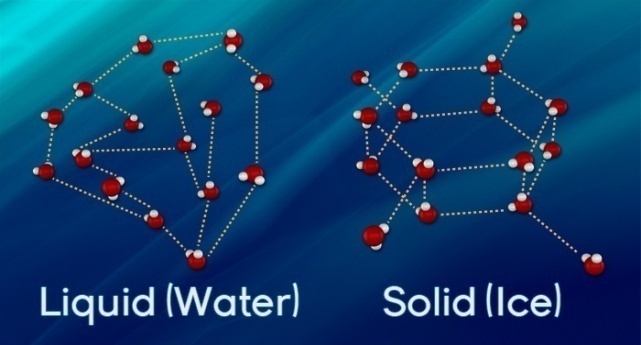
· When water freezes, water molecules form a crystalline structure maintained by hydrogen bonding.
· Solid water, or ice, is less dense than liquid water.
· Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart, which lowers the density.
· For other liquids, solidification when the temperature drops includes the lowering of kinetic energy, which allows molecules to pack more tightly and makes the solid denser than its liquid form.
· Because ice is less dense than water, it is able to float at the surface of water.
The formation of hydrogen bonds is an important quality of liquid water that is crucial to life as we know it. As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids, and since living things have a high water content, understanding these chemical features is key to understanding life. In liquid water, hydrogen bonds are constantly formed and broken as the water molecules slide past each other. The breaking of these bonds is caused by the motion (kinetic energy) of the water molecules due to the heat contained in the system. When the heat is raised as water is boiled, the higher kinetic energy of the water molecules causes the hydrogen bonds to break completely and allows water molecules to escape into the air as gas (steam or water vapor). On the other hand, when the temperature of water is reduced and water freezes, the water molecules form a crystalline structure maintained by hydrogen bonding (there is not enough energy to break the hydrogen bonds). This makes ice less dense than liquid water, a phenomenon not seen in the solidification of other liquids.
Water's lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: the water molecules are pushed farther apart compared to liquid water. With most other liquids, solidification when the temperature drops includes the lowering of kinetic energy between molecules, allowing them to pack even more tightly than in liquid form and giving the solid a greater density than the liquid.
The low density of ice, an anomaly, causes it to float at the surface of liquid water, such as an iceberg or the ice cubes in a glass of water. In lakes and ponds, ice forms on the surface of the water creating an insulating barrier that protects the animals and plant life in the pond from freezing. Without this layer of insulating ice, plants and animals living in the pond would freeze in the solid block of ice and could not survive. The detrimental effect of freezing on living organisms is caused by the expansion of ice relative to liquid water. The ice crystals that form upon freezing rupture the delicate membranes essential for the function of living cells, irreversibly damaging them. Cells can only survive freezing if the water in them is temporarily replaced by another liquid like glycerol.
| 
|
| Ice Density Hydrogen bonding makes ice less dense than liquid water. The (b) lattice structure of ice makes it less dense than the freely flowing molecules of liquid water, enabling it to (a) float on water. |
Water's polarity
Water's polarity is responsible for many of its properties including its attractiveness to other molecules:
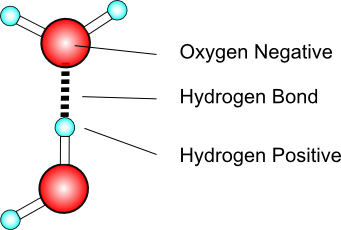
· The difference in electronegativities between oxygen and hydrogen atoms creates partial negative and positive charges, respectively, on the atoms.
· Water molecules attract or are attracted to other polar molecules.
· Molecules that do not dissolve in water are known as hydrophobic (water fearing) molecules.
One of water's important properties is that it is composed of polar molecules. The two hydrogen atoms and one oxygen atom within water molecules (H2O) form polar covalent bonds.
While there is no net charge to a water molecule, the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen, contributing to water's properties of attraction. Water's charges are generated because oxygen is more electronegative, or electron loving, than hydrogen. Thus, it is more likely that a shared electron would be found near the oxygen nucleus than the hydrogen nucleus. Since water is a nonlinear, or bent, molecule, the difference in electronegativities between the oxygen and hydrogen atoms generates the partial negative charge near the oxygen and partial positive charges near both hydrogens.
As a result of water's polarity, each water molecule attracts other water molecules because of the opposite charges between them, forming hydrogen bonds. Water also attracts, or is attracted to, other polar molecules and ions, including many biomolecules, such as sugars, nucleic acids, and some amino acids. A polar substance that interacts readily with or dissolves in water is referred to as hydrophilic (hydro- = "water"; -philic = "loving"). In contrast, nonpolar molecules, such as oils and fats, do not interact well with water, as shown in . These molecules separate from it rather than dissolve in it, as we see in salad dressings containing oil and vinegar (an acidic water solution). These nonpolar compounds are called hydrophobic (hydro- = "water"; -phobic = "fearing").
The hydrogen bond
Water makes use of the hydrogen bond, a type of intermolecular force experienced when hydrogen is attracted to the electronegative atoms nitrogen, oxygen, or fluorine. Hydrogen bonding is the strongest intermolecular force.
Дата добавления: 2018-11-25; просмотров: 1872;
