Rate Laws of Elementary Steps
· The rate laws of the all-elementary steps determine the overall rate law of the reaction.
· The rate law of an elementary step is determined by its molecularity:
1. iMolecular processes are first order,
2. Bimolecular processes are second order, and
3. Termolecular processes are third order.
Rate Laws of Multistep Mechanisms
Rate-determining step: is the slowest of the elementary steps.
Therefore, the rate-determining step governs the overall rate law for the reaction.
For instance, the following reaction occurs in two steps:
· NO2 (g) + CO (g) NO (g) + CO2 (g)
Step 1. NO2 (g) + NO2 (g) k1 NO3 (g) + NO (g) (slow)
Step 2. NO3 (g) + CO (g) k2 NO2 (g) + CO2 (g) (fast)
K2 >>>K1 , NO3 slowly produced in step 1 is immediately consumed in step 2
The rate determining steps is step 1 (slowest step):
R= k1*[NO2]2 the rate law predicted by this mechanism agrees with the one observed experimentally.
Mechanisms with an Initial Fast Step
It is possible for an intermediate to be a reactant.
Consider:
2NO (g) + Br2(g) 2NOBr(g)
The experimentally determined rate law is:
Rate = k*[NO]2*[Br2]
The termolecular mechanism is be ruled out since it is not probable
Consider another mechanism:
k1
Step 1: NO (g) + Br2 (g) NOBr2 (g) (fast)
k-1
k2
Step 2: NOBr2 (g) + NO (g) 2NOBr (g) (slow)
for which the rate law is (based on Step 2):
Rate = k2*[NOBr2]*[NO].
The rate law should not depend on the concentration of an intermediate because intermediates are usually unstable.
Assume NOBr2 is unstable, so we express the concentration of NOBr2 in terms of NOBr and Br2 assuming there is an equilibrium in Step 1
We have by definition of equilibrium:
k1*[NO]*[Br2] = k-1*[NOBr2] therefore:
[NOBr2] = k1/k-1*[NO]*[Br2
Rate = k2*[NOBr2]*[NO]
The overall rate law becomes:
Rate = k2*k1/k-1*[NO]*[Br2]*[NO] = k*[NO]2*[Br2].
Catalysis
· A catalyst changes the rate of a chemical reaction by lowering the activation energy
Catalysts can operate by increasing the number of effective collisions.
That is, from the Arrhenius equation: catalysts increase k by increasing A or decreasing Ea.
k = Ae-Ea/RT
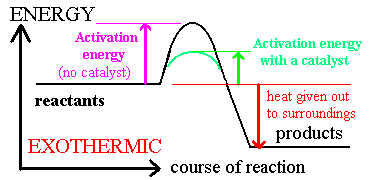
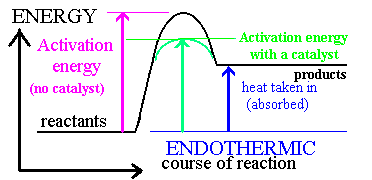
There are two types of catalyst:
Homogeneous
Heterogeneous
Homogeneous Catalysis
The catalyst and reaction is in one phase.
Example:
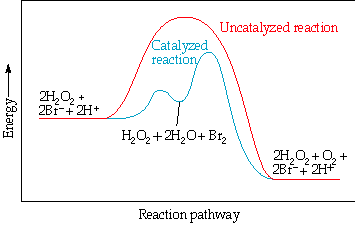
Hydrogen peroxide decomposes very slowly:
2H2O2 (aq) 2H2O (l) + O2 (g).
In the presence of the bromide ion, the decomposition occurs rapidly:
2Br - (aq) + H2O2 (aq) + 2H+ (aq) Br2 (aq) + 2H2O (l)
Br2 (aq) is brown.
Br2 (aq) + H2O2 (aq) 2Br - (aq) + 2H+ (aq) + O2 (g).
Br- is a catalyst because it can be recovered at the end of the reaction.
The mechanism pathway with catalyst is different from that without catalyst because the catalyst (Br-) added an intermediates (Br2 (aq)) to the reaction.
Дата добавления: 2018-11-25; просмотров: 630;
