III. A common catabolic pathway.
Acetyl-CoA undergoes oxidation in the cycle of di- and tricarboxylic acids (Krebs cycle). Oxidation is accompanied by the formation of reduced forms of NADH + H+ and FADH2. These are the primary donors of hydrogen for the electron transport chain. Reactions of the common catabolic pathway occur in the matrix of mitochondria. Reduced coenzymes transfer hydrogen directly to the components of the respiratory chain which are located in the inner membrane of mitochondria, where ATP is produced. There is a transfer of electrons from reduced nucleotides to oxygen (through the respiratory chain). It is accompanied by the formation of water. This electron transport is associated with ATP synthesis during oxidative phosphorylation. At this stage, 2/3 energy of nutrients are released.
Thus, after the formation of pyruvic acid further path of breakdown of substances to CO2 and H2O occurs identically in the common catabolic pathway.

Metabolites of the common catabolic pathway are precursors in the synthesis of a number of substances in the body. For example, pyruvate is a precursor for glucose and acetyl-CoA is a precursor for fatty acids.
6.2. BIOENERGETICS
Metabolism is divided into two categories: catabolism and anabolism. One of the main functions of catabolism is receiving chemical energy contained in nutrients and the usage of this energy to provide necessary functions.
The energy of oxidized substances is used mainly for the synthesis of ATP from ADP. ATP is the universal source of energy in the body.
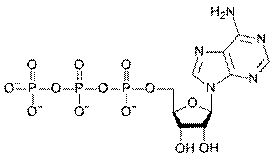
If the energy released during the hydrolysis reaction of the substance, exceeds 30 kJ/mole, the hydrolysable bond is called a high-energy (energy-rich) bond. The energy of ATP hydrolysis is about 50 kJ/mole. In formula the energy-rich bond is denoted by ~ (tilde).
Types of energy-rich compounds: pyrophosphates (ATP), phosphoguanidines (creatine phosphate), acyl phosphate (1,3 bisphosphoglycerate), enol phosphates (phosphoenol pyruvate), thiol esters (acetyl-CoA).
Cell energy supply can occur in anaerobic conditions:
C6H12O6 = 2 C3H6O3 + 65 kJ/mole
One way of ATP synthesis from ADP is substrate level phosphorylation.It is ATP formation at the expense of other high-energy compound:
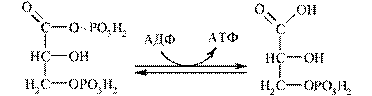
1,3-bisphosphoglycerate 3-phosphoglycerate
The use of oxygen by cells opens the possibility for a more complete Tissue respiration is a set of oxidation reactions of substrates in living cells, accompanied by the consumption of molecular oxygen and leading to the releasing of carbon dioxide and water and formation of biological energy.
For the first time the essence of breath was explained by Antoine Laurent Lavoisier (1777); he drew attention to the similarity between the combustion of organic substances outside the body and the respiration of animals. In the body, oxidation occurs at relatively low temperature in the presence of water, and its velocity is regulated by metabolism.
Let’s consider the reaction of glucose oxidation:
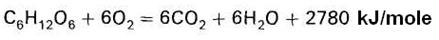
In the body, this process is multistage. Carbon is converted into carbon dioxide by the oxygen of the oxidized substance and the oxygen of water. This reaction involves hydrogen acceptors, which carry hydrogen on oxygen. Oxygen is used for the synthesis of water from the hydrogen of oxidized substrates.
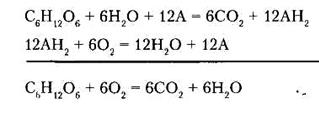
The other substances are oxidized in the same way. Kidneys, brain, liver are characterized by the highest rate of tissue respiration; and the skin, muscles (at rest) - the smallest one.
The main way of ATP synthesis from ADP is oxidative phosphorylation. This is the synthesis of ATP from ADP and inorganic phosphate, which occurs due to the energy released by oxidation of organic substances in the process of cellular respiration. It is coupling of respiration and phosphorylation.
ADP + H3PO4 + energy ® ATP + H2O
6.3. ORGANIZATION AND OPERATION
OF THE RESPIRATORY CHAIN
Oxidation of substrates in the process of respiration can be represented as the transfer of electrons and protons (i.e. hydrogen atoms) of organic substances to oxygen. This process involves a series of intermediate transporters which form the respiratory chain.
Respiratory chain (electron transport chain) is the system of transmembrane proteins and electron carriers that transfer electrons from substrates to oxygen. In eukaryotic cells the respiratory chain is located in the inner membrane of mitochondria.
Reduced NAD is a universal donor of hydrogen atoms to the respiratory chain. The interaction of NAD and NADP with hydrogen atoms is a reversible addition of hydrogen atoms. Two electrons and one proton are included in the molecule of NAD (NADP); and the second proton remains in the environment.
Another primary source of hydrogen atoms and electrons is reduced flavoprotein (FAD or FMN).
Reduced forms of these cofactors are able to carry hydrogen and electrons to the mitochondrial respiratory chain.
The components of the respiratory chain are embedded in the mitochondrial membrane in the form of four protein- lipid complexes (Fig. 19).
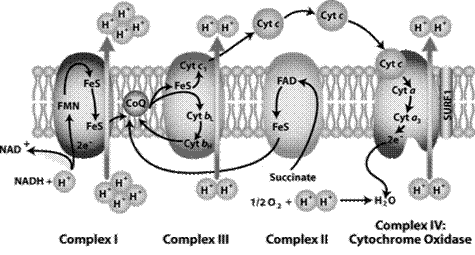
|
| Fig. 19. Mitochondria lrespiratory chain |
Complex I (NADH dehydrogenase, NADH-CoQ reductase)contains FMN and iron-sulfur protein FeS (non-heme iron). Fe can be in Fe2+ and Fe3+-form. Iron-sulfur protein is involved in the redox process. Complex I oxidizes NADH and transfers 2 electrons from it to KoQ. Complex I also pumps four protons from the matrix into the intermembrane space of mitochondria.
KoQ (ubiquinone) is a derivative of benzoquinone. It is not large lipophilic molecule. Ubiquinone moves into the lipid bilayer of the membrane and provides transfer of electrons between complexes I - III and II - III.
Complex II (succinate dehydrogenase, succinate-CoQ reductase) contains FAD and iron-sulfur protein. It provides input of additional electrons into the chain from succinate oxidation.
Complex III (QH2 dehydrogenase, CoQ-cytochrome C reductase) contains cytochrome b and с1 and iron-sulfur protein. Cytochromesare hemoproteins which prosthetic groups are close to heme of hemoglobin (in the cytochrome b it is identical). Complex III transfers electrons from ubiquinone to cytochrome c and pumps 2 protons into the intermembrane space.
Complex IV (cytochrome oxidase) is composed of cytochromes a and a3, which, in addition to heme, contain copper ions. Cytochrome а3is the terminal component of respiratory chain. Complex IV catalyzes the transfer of electrons from molecules of cytochrome to O2 and pumps four protons into the intermembrane space. Cytochrome oxidase carries out the oxidation of cytochrome c and formation of water.
300-400 ml of water per day (metabolic water) are formed in the humans mitochondrial respiratory chain.
The components of the mitochondrial respiratory chain are in descending order of redox potential. Electron transport in the respiratory chain occurs on a gradient of redox potential and is a source of energy for proton transfer. As a result, difference in the concentrations of protonsoccurs on the sides of the membrane; while the difference in electrical potential from the "plus" sign - on the outer surface. Electrochemical potential of protons makes protons move in the opposite direction - from the outer surface inward. However, the membrane is impermeable to them, except in areas where the enzyme is a proton ATP synthase (Fig. 20). It consists of two parts - stator and rotor.
The stator consists of three α-subunits and three β-subunits - they participate directly in the synthesis of ATP from ADP and phosphate. δ- subunit joins them, and together they form the F1- subunit.
The rotor consists of gamma and epsilon subunits.
The stator is held in the membrane, and the rotor is rotated by the energy of the protons.
In the stator there is a proton channel (F0). It consists of two parts which are shifted relative to one another. Proton passes one half of the channel, and then it enters into the second half of the channel on the rotating rotor. The difference of electrochemical potentials resulting during the movement of protons through the channel activates the ATP synthase, which catalyzes reaction:
ADP + H3PO4 ® ATP + H2O
Chemiosmotic hypothesis of energy transformation in living cells was proposed by P. Mitchell in 1960 to explain the molecular mechanism of coupling of electron transport and ATP formation in respiratory chain. For the development of research in the field of bioenergetics P. Mitchell was awarded the Nobel Prize in 1978.
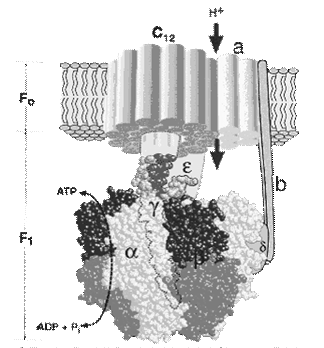
|
| Fig. 20. The structure of the proton ATP-synthase. |
There are only three sites in the respiratory chain where the transfer of electrons is connected with energy storage, sufficient for the formation of ATP.
The coefficient of phosphorylation is the ratio of ATP formed by the absorbed oxygen: ATP/O, or ratio refers to the number of inorganic phosphate molecules utilized for ATP generation for every atom of oxygen consumed: P/O. The maximum value of the coefficient of phosphorylation is 3 (if the oxidation reaction goes with the participation of NAD), and 2 (if the oxidation of substrate goes with the participation of FAD). Actually obtained value is smaller (2.5 and 1.5). That is the process of respiration is not completely coupled with phosphorylation. The degree of coupling depends mainly on the integrity of the mitochondrial membrane.
Formed ATP is transported from the matrix on the outer side of the membrane with the participation of the ADP-ATP-translocase, and then goes into the cytosol. At the same time, the same translocase carries ADP in the reverse direction, from the cytosol into the mitochondria matrix.
The total content of ATP is 30-50 grams in the body, but the average life expectancy of ATP molecule is less than 1 minute. A person synthesizes 40-60 kg of ATP and the same amount splits per day.
At each contraction of the heart muscle, about 2% of the available ATP in it are consumed. All ATP would be expended over 1 minute, if there was no its regeneration. In the formation of a blood clot in the coronary artery, the supply of oxygen to the cells is ceased; and the regeneration of ATP is respectively blocked and cells die (myocardial infarction).
Increase of concentrations of ADP leads to acceleration of respiration and phosphorylation. The intensity of respiration of mitochondria on the concentration of ADP is called respiratory control.
We use cell energy charge (CEC) to assess the impact of adenine nucleotide over the processes of metabolism:
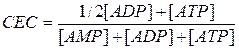
Normally CEC = 0.7-0.8: the rate of ATP formation equals the rate of its use and adenylic system is full of energy.
When CEC<0.7 the formation of ATP is accelerated by increasing the rate of reactions of common way catabolism.
If CEC = 1, then the processes of ATP synthesis are inhibited, and its use is accelerated.
Hypoenergy states are divided into:
- alimentary (starvation, vitamin deficiency);
- hypoxic:
- disorder of oxygen entry in the blood (pulmonary hypoxia),
- disorder of oxygen transport to tissues (hemodynamic (blood loss, shock, heart defects) and hemoglobin hypoxia (Hb pathology, blocking it with poisons));
- mitochondrial (difficulties of oxygen use in cells). This is disruption of mitochondria function by inhibitors of respiratory chain enzymes, releasers of oxidation and phosphorylation, membrane-acting agent.
At complete starvation, food reserves of the body are enough for several weeks, and oxygen is enough only for 2-3 minutes. Therefore, hypoxia is the most common cause of hypoenergy states, and hypoxia of the brain is the direct cause of death. Therefore, the measures for restoring the oxygen supply take the leading place among the resuscitation procedures.
6.4. UNCOUPLING of oxidation FROM phosphorylation
Uncouplers are lipophilic substances that can accept protons, and carry them through the inner mitochondrial membrane, but not passing its proton channel.
Natural uncouplers are products of peroxidation of lipid, fatty acids with a long chain, and high doses of thyroid hormones.
Artificial uncouplers are dinitrophenol, ether, derivatives of vitamin K, anesthetics, antibiotics (gramicidin, valinomycin)
Consider the example of uncoupling mechanism in dinitrophenol. It easily diffuses across the mitochondrial membrane in both ionized and a non-ionized forms and can carry hydrogen ions across the membrane. Therefore, 2,4-dinitrophenol destroys ΔpH of mitochondrial membrane. Oxygen consumption and substrate oxidation are continuing, but ATP synthesis is impossible. Since the energy of oxidation in uncoupling is dissipated in the form of heat, the uncouplers increase body temperature (pyrogenic action).
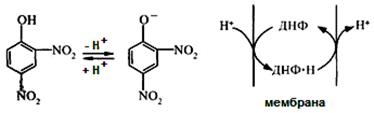
There is a particular tissue, specialized in heat production by uncoupling of respiration from oxidative phosphorylation. This is brown adipose tissue. It has such name because of a large number of mitochondria (they are brown). About 10% of all proteins of these mitochondria accounts for the so-called uncoupling protein. Brown adipose tissue is involved in maintaining body temperature.
Biological oxidation which is not accompanied with the storage of energy is called the free (uncoupling) oxidation. It accounts for 5-10% of oxygen entering the body. It is out of mitochondria, mostly in the endoplasmic reticulum, so sometimes this process is called microsomal oxidation(microsomes are fragments of ER).
One of the functions of free oxidation is transformation of natural or unnatural substrates, called xenobiotics.
Free oxidation takes place with the participation of oxygenases. Oxygenases are enzymes of oxidoreductases class; they catalyze the oxidation of substrates by including one oxygen atom (monooxygenases) or two oxygen atoms (dioxygenases) in their molecules.
Oxygenases work in the multienzymatic complex, embedded in the membrane. Multienzymatic complex consists of 3 components: flavin dehydrogenases, iron-sulfur protein, cytochrome P450.
Cytochrome P450 is a group of enzymes related to hemoproteins. Cytochrome P450 system participates in the oxidation of both endogenous (steroids, bile acids, unsaturated fatty acids) and exogenous substances which are called xenobiotics (xeno - incompatible, bios - life), for example, medicines, poisons and drugs.
Oxygen and reduced respiratory carriers (usually NADPH) are also involved in reactions of free oxidation. Cytochrome P-450 is an electron acceptor.
RH + O2 + NADPH + H+ ® ROH + NADP+ + H2O
Hydroxylation of xenobiotic makes it more soluble, facilitates the following destruction and elimination from the body.
6.5. GENERATION of free radicals in cells
Reactive oxygen species (ROS)are compounds in which oxygen has an unpaired electron.
ROS are formed in the following cases: when the conditions of the respiratory chain action are changed (e.g., during hypoxia); under UV rays; during the interaction of oxygen with metal ions of variable valence (iron); during the spontaneous oxidation of some substances; during the participation of the enzymes xanthine oxidase or NADPH oxidase. Under these conditions, asuperoxide anion of oxygen О2×-is formed, then hydrogen peroxide Н2О2andhydroxyl radical HO×. Reactive oxygen species are causing lipid peroxidation.It is a process leading to severe damage of membranes, proteins and DNA damage.
Inactivation of reactive oxygen species in cells is under the influence of the antioxidant system. It consists of several antioxidant enzymes and low molecular antioxidants (vitamin C, glutathione, vitamin E, etc.).
Superoxide dismutase (SOD) converts superoxide anion of oxygen into hydrogen peroxide Н2О2:
2 O2-.+ 2Н+® H2O2 + O2.
Catalase is hemin enzyme, containing Fe3+. It catalyzes the reaction of hydrogen peroxide splitting. In this reaction water and oxygen are formed:
2 H2O2® O2 + 2H2О.
The highest catalase activity in the body is typical of the liver. There is a lot of catalase in erythrocytes. There it protects the heme of hemoglobin from oxidation.
Peroxidase is hemin enzyme which reduces hydrogen peroxide to water, while another substance is oxidized.
2 H2O2® 2H2О + RO2.
Peroxidase is able to split other peroxides, converting them to alcohols. Peroxidase activity is found in the liver, kidneys, and neutrophilic leukocytes.
Antioxidants are biologically active substances that interact with free radicals and prevent the process of free radical oxidation of organic substances in the body.
Vitaminswhich have antioxidant properties are C, E, A, P. Glutathione, taurine (2-aminoetansulfonic acid), dipeptide carnosine are also reveal antioxidant properties.
Complete suppression of peroxide processes in the tissues seems inappropriate. Free radicals induce apoptosis and are involved in the formation of cellular immunity, stimulate phospholipases, thereby participating in the synthesis of eicosanoids.
However, the enhanced generation of free radicals accompanies pathological conditions (Parkinson's disease, Alzheimer's disease) and the process of biological aging.
6.6. REACTIONS OF THE COMMON CATABOLIC PATHWAY
6.6.1. Oxidative decarboxylation of PYRUVATE
This is a multistep process, which is catalyzed by the pyruvate dehydrogenase complex.It is a mitochondrial multienzymatic complex, connected to the inner membrane from the matrix. Pyruvate comes to the complex from the matrix and acetyl-CoA and NADH are also exempted there.
Pyruvate dehydrogenase complex consists of three enzymes (pyruvate dehydrogenase (E1), acetyl transferase (E2), dehydrogenase of dihydrolipoic acid (E3)) and five coenzymes (NAD, FAD, thiamine pyrophosphate (TPP), lipoic acid, coenzyme A). Thiamine pyrophosphate is attached to pyruvate decarboxylase (E1); lipoic acid - to acetyltransferase (E2), FAD - to dehydrogenase of dihydrolipoic acid (E3). Coenzyme A and NAD+ are in a free state (in dissolved state).
The composition of the pyruvate dehydrogenase complex consists of about three dozen molecules of E1 and E2 and 10 molecules of E3. It is bigger than ribosome. The complex acts as a conveyer: the intermediates are not released into solution, and are passed from enzyme to enzyme.
Pyruvate dehydrogenase system is characterized by a large negative redox potential, which ensures the formation of high-energy thioester bond in acetyl-CoA.
The first reactionis catalyzed by E1; substrates are pyruvate and dehydrolipoic acid, which is a prosthetic group of E2. The carboxyl group splits from pyruvate; then СО2 is formed; but acetyl residue combines with the sulphur atom of lipoic acid in the acetyl transferase. Acetyllipoat-E2 is formed.

 CH3-C-COOH + E1-TPP ® E1-TPP-CH-CH3 + CO2 (1)
CH3-C-COOH + E1-TPP ® E1-TPP-CH-CH3 + CO2 (1)
O OH


 E1-TPP-CH-CH3 + S S ® E1-TPP + CH3-C-S SH (2)
E1-TPP-CH-CH3 + S S ® E1-TPP + CH3-C-S SH (2)
OH -E2 O -E2
In the second reaction acetyl transferase (E2) catalyzes the transfer of acetyl residue, coupled with its own prosthetic group, to coenzyme A. The products of this reaction are dihydrolipoic acid in the E2 and acetyl-CoA.

 CH3-C-S SH + HS~CoA ® CH3-CO~CoA + HS SH (3)
CH3-C-S SH + HS~CoA ® CH3-CO~CoA + HS SH (3)
O -E2 -E2
In the third reaction the dehydrogenation of dihydrolipoic acid in the acetyl transferase takes place under the influence of the enzyme E3 (dehydrogenase of dihydrolipoic acid). E3 contains FAD. FAD transfers hydrogen to NAD. So NADH, H+ and dehydrolipoic acid in the E2 are formed. The latter enzyme re-enters the oxidative decarboxylation of pyruvate.

 HS SH + FAD-E3 ® S S + FAD×H2-E3 (4)
HS SH + FAD-E3 ® S S + FAD×H2-E3 (4)
-E2 -E2
FAD×H2-E3 + NAD+ ® FAD-E3 + NADH + H+ (5)
The overall equation of the process:
СH3 - CО - COOH + HS –CoA + NAD+ ® СH3 – CО - S - KoA + NADN + Н+ + СО2.
Acetyl-CoA (a product of the third reaction) is then oxidized in the Krebs cycle. Hydrogen from NADH (the product of the fifth reaction) enters the respiratory chain, where ATP is formed. Energy output of the oxidative decarboxylation of pyruvate is 3 ATP.
According to the mechanism of feedback inhibition, the work of pyruvate dehydrogenase complex is inhibited by end-products of oxidative decarboxylation - acetyl-CoA, NADH+H+, and ATP. Pyruvic acid increases the activity of complex.
There is also a regulation by hormones: insulin increases the activity of the complex, but glucagon decreases it.
6.6.2. CITRIC acid CYCLE
Citric acid cycle (Krebs cycle, cycle of di- and tricarboxilic acids)was opened by British biochemist Hans Krebs; he received the Nobel Prize for this outstanding discovery in 1953.
Krebs cycle is a way of oxidation of molecules of acetyl-CoA which are formed from the most carbohydrates, fatty acids and amino acids during the process of catabolism.
Citric acid cycle takes place in the matrix of mitochondria. Its steps are:
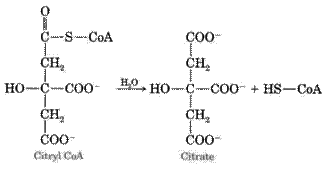
1. The condensation reaction of acetyl-CoA with oxaloacetate under the action of citrate synthase with the formation of citric acid:
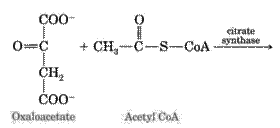
2. Dehydration of citric acid goes with the formation of cis-aconitic acid. It adds water molecule and becomes isocitric acide (isocitrate). The enzyme catalyzing this reaction is aconitate hydratase (aconitase):
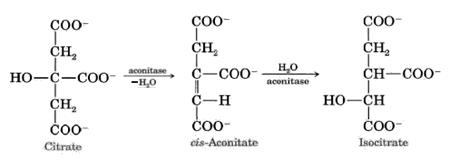
3. The next step is the reaction of dehydrogenation and decarboxylation of isocitric acid in the presence of NAD-dependent isocitrate dehydrogenase. This is the limiting stage of the Krebs cycle; as a result, a-ketoglutarate is formed. NAD-dependent isocitrate dehydrogenase is activated by ADP and ions of Mg2+:
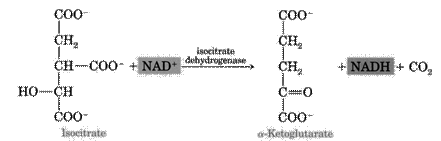
4. Oxidative decarboxylation of a-ketoglutaric acid goes with the participation of a-ketoglutarate dehydrogenase complex, which includes five coenzymes: thiamine pyrophosphate, lipoic acid, coenzyme A, FAD and NAD. It leads to the formation of the succinyl-CoA. It is energy-rich compound.
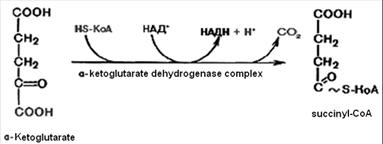
5. Succinyl-CoA with the participation of GDP and inorganic phosphate is converted into succinate (succinic acid). At the same time GTP is formed. This is substrate level phosphorilation. This reaction is catalyzed by succinyl-CoA-synthetase:
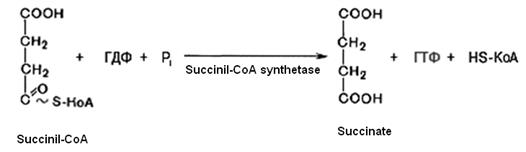
GTP + ADP ® ATP + GDP
6. Dehydrogenation reaction of succinate goes with the participation of succinate dehydrogenase (includes coenzyme FAD) which is attached to the inner mitocondrial membrane. Fumarate is formed.
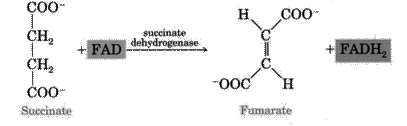
7. The next reaction is hydration of fumaric acid which leads to the formation of L-malate. The enzyme is fumarase.
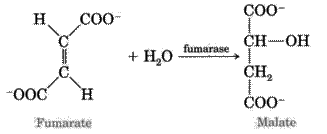
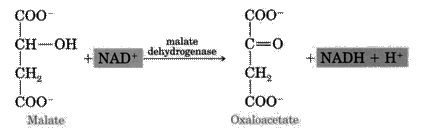
8. Under the influence of mitochondrial NAD-dependent malate dehydrogenase L-malate is oxidized to oxaloacetate and, thus, the Krebs cycle is closed.
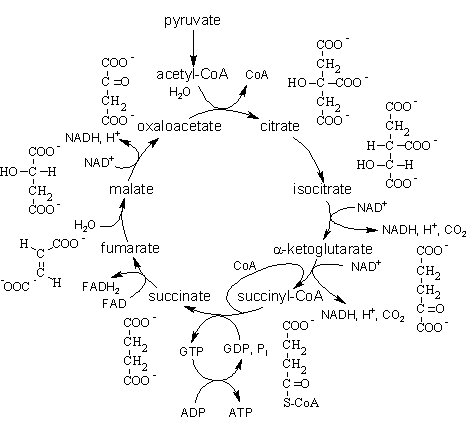
In the Krebs cycle 3 molecules of NADH + H+ (reactions 3, 4, 8) are formed. Therefore, the electron transport chain synthesizes 3x3 = 9 ATP molecules.
1 molecule FADH2 is formed in reaction (6), therefore, 2 molecules of ATP in the respiratory chain are formed.
And 1 molecule of ATP is formed from GTP (reaction 5) in the trans-phosphorilation reaction.
TOTAL: 9 + 2 + 1 = 12 ATP.
The Krebs cycle is regulated by a mechanism of negative feedback inhibition with the participation of allosteric enzymes. NADH inhibit NAD-dependent dehydrogenases of Krebs cycle. With a decrease in ATP consumption activity of the respiratory chain is reduced (respiratory control), the concentration of NADH in the cell increases, and inhibition of reactions 3, 4, and 8 of the Krebs cycle decreases the activity of the Krebs cycle as a whole.
We use cell energy charge (CEC) to assess the influence of adenine nucleotide on the metabolism:
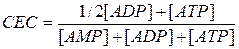
Normally CEC = 0.7-0.8: the rate of ATP formation equals the rate of its use and adenylic system is full of energy.
When CEC<0.7 the formation of ATP is accelerated by increasing the rate of reactions of common catabolic pathway.
If CEC = 1, then the processes of ATP synthesis are inhibited, and its use is accelerated.
Дата добавления: 2018-09-24; просмотров: 1754;
