Immobilized cell reactors
Whole cell immobilization has been defined as "the physical confinement or localization of intact cells to a certain defined region of space with preservation of some desired catalytic activity". Many microorganisms own the capability to adhere to different kinds of surfaces in nature on which way are in close proximity to nutrients and easy realize a food supply.
However, many biotechnological processes need to be carried out using immobilization of biocatalysts. Thereby several different techniques and support materials have been proposed for the cell immobilization in vitro. Figure below illustrates basic methods for immobilization. These techniques can be divided into four major groups based on the physical mechanism causing immobilization: physical entrapment within a porous matrix, attachment or adsorption to a pre-formed carrier, self aggregation by flocculation (natural) or cross linking agents (artificially induced) and cell contained behind barrier.
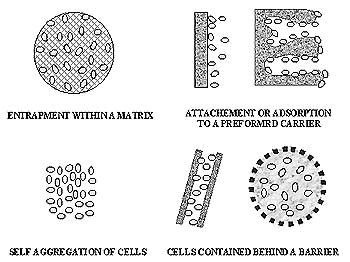
| Figure: Principal methods of cell immobilization (source: http://www.rcub.bg.ac.yu/~todorom/tutorials/rad15.html, 2009) |
By using cell immobilization methods, cell concentrations of 50-70 g/L can be achieved in the reactor. By contrast in batch reactors cell concentration doesn't exceed 48 g/L. Advantages of immobilized cell systems are:
- Control over cell density in the medium
- High cell density
- Smaller reactor volume
- Higher productivity
- Flexibility with reactor configuration (fixed bed, fluidized bed, trickle bed,...)
- Maximum reaction rates
- Simpler nongrowth media when stationary-phase cells are immobilized
Some problems of immobilized cell reactors which may arise with anaerobic, gas-producing fermentations are accumulation of the bubbles in the immobilisation matrix and mass transport limitation of substrate and products.
The immobilized whole cell system is an alternative to enzyme immobilization. Unlike enzyme immobilization, where the enzyme is attached to a solid support (such as calcium alginate), in immobilized whole cell systems, the target cell is immobilized. Such methods may be implemented when the enzymes required are difficult or expensive to extract, an example being intracellular enzymes. Also, if a series of enzymes are required in the reaction; whole cell immobilization may be used for convenience.This is only done on a commercial basis when the need for the product is more justified.
Advantages. Multiple enzymes may be introduced into the reaction, thus eliminating the need for immobilization of multiple enzymes. Furthermore, intracellular enzymes need not be extracted prior to the reaction; they may be used directly.
Disadvantages. Some enzymes may be used for the metabolic needs of the cell, leading to reduced yield.
Aim: To fix the engineering bacteria from suspended batch cultivation to the inner surface of device, namely cell immobilization.
2. Methods of cell immobilization
Cell immobilization is a technique to fix cells in a suitable matrix. In the past, various cells have been immobilized. A number of methods have been founded and developed base on various principles: entrapment, ion exchange adsorption, porous ceramics, and even covalent bonding. After studying literatures and asking senior students for help, we decided to explore three methods to immobilize our engineering bacteria:
2.1 Immobilizing cells into calcium alginate beads
Calcium alginate gel apply to entrapping cells has advantageous properties such as high mechanical strength, inexpensive cost, mild and simple operation conditions and good biocompatibility. Therefore, this method is widely used in cell immobilization. In this project, we chose making calcium alginate beads as our first trial of immobilization experiments.
2.2 Immobilizing cells into intra-hollow Ca-alginate capsules
Embedding cells in intra-hollow Ca-alginate capsules is a technique derives from calcium alginate immobilization system. This method not only covers most of the advantages of calcium alginate entrapment, but also overcomes many difficulties. Substrates and oxygen can easier transfer into intra-hollow capsules than into calcium alginate beads. Moreover, while cells can only grow on the beads' surface and in the gel holes, the grow space of cells largely broaden in capsules since they enwrap liquid interior medium.
2.3 Immobilizing cells into NaCS-PDMDAAC microcapsules
Embedding cells into NaCS-PDMDAAC microcapsules is one kind of new and promising immobilization technique, which has well biocompatibility and excellent light transmission. However, the preparation of NaCS is difficult and time-consuming. Through experiments, we also found that mechanical strength of NaCS-PDMDAAC microcapsules was too low for our project. When the microcapsules were transferred from one culture to another, they broke easily. That indicates if our engineering bacteria were embedded in NaCS-PDMDAAC microcapsules, they cannot be well fixed in some kinds of continuous culture systems, such as our tubes.

Перспективы развития микробиологической биотехнологии – для самостоятельной проработки.
Роль белков как компонента пищи – как составная часть вопроса об «одноклеточном» белке – для самостоятельной проработки.
Дата добавления: 2015-07-14; просмотров: 1733;
