Synthesis
4-(Carboxymethyl)-2,2,6,6-tetramethylpiperidine chloride was synthesized synthesized according to the following scheme:
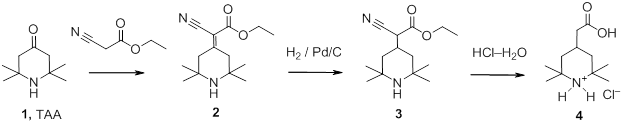 |
The first phase is The Knoevenagel condensation:
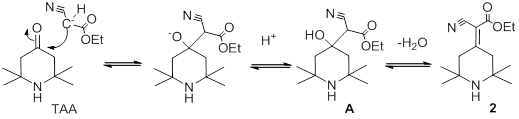 |
The product was selective Hydrogenated:
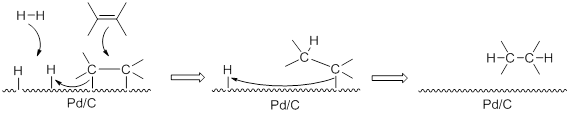 |
Next step is acid hydrolysis of the nitrile group and a derivative of malonic acid:
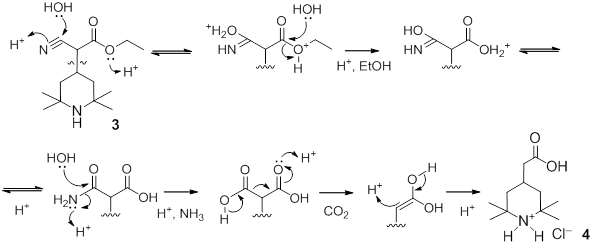
As the result, fragment cyanoacetic ester is transformed into the target substituted acetic acid.
Experimental
2,2,6,6- Tetramethyl-4- (carboethoxycyanomethylene)piperidine (III). A mixture of 100 g of (II), 72.2 g of ethylcyanoacetate, and 20 g of ammonium acetate was refluxed in 200 ml of benzene for 1 h with azeo- tropic removal of water. The reaction mixture was cooled, treated with excess 50% potassium carbonate, and extracted with benzene. After removal of solvent, 150 g (97%) of technical grade (III) was obtained.
Pure (III) is a colorless crystalline powder withmp 57-58° (from ethyl acetate) and bp 128-130° (1.5 mm). It is readily soluble in the usual organic solvents. Found %r С 67.23; H 8.76. N 11.23. C14H22N202. Calculated %: C. 67.17; H 8.85; N 11.19. The hydrochloride is obtained as colorless Crystals with mp 210- 211° and is soluble in water and alcohol. Foimd %: Cl 12.34; N 9.99. C14H22N2(V HC1. Calculated %: Cl 12.33 N 9.77
2,2,6,6-Tetramethyl-4- (carboethoxycyanomethyl)piperidtne (IV). Palladium (5%) on calcium carbonate (total weight 21.2 g) was added to 150 g of technical (Ш) in 900 ml of alcohol and hydrogenated in an autoclave at 18-20* and 15 atm. After the usualworkup, 145 g @7.3%) of technical (IV) was obtained as a viscous yellow mass which crystallized on standing. Pure (IV) is a colorless crystalline powder with mp 56-58°
(from ethyl acetate) and bp 135-140° (4 mm) and is readily soluble in the usual organic solvents. Found %:
С 66.53; H 9.36; N 11.01. C14H24N202. Calculated %: С 66.63; H 9.55; N 11.10. The hydrochloride is obtained as colorless crystals with mp 217-218° and is soluble in water and alcohol. Found %: Cl 12.49;
N 9.96. C14H24N2021HC1. Calculated %: Cl 12.41; N 9.701
Methyl 2,2,6,6-Tetramethylpiperidyl-4-acetate chloride (VI). Technical (IV) (145 g) was refluxed for 15 h with 1100 ml of hydrochloric acid. The reaction mixture was evaporated, the residual water was removed by azeotropic distillation with benzene, the residue was dissolved in 185 ml of methanol, and 70 ml of concentrated sulfuric acid was added dropwise during cooling with ice. The mixture was stirred under re- N flux for 8 h, treated with excess 50% potassium carbonate, and extracted with dichloroethane. After removal of the solvent the residue was distilled and the fraction boiling at 114-116° (11 mm) was collected V: to obtain 67 g (54.6%) of (VI). (VI) was a clear liquid which was readily miscible with the usual organic solvents. Found %: C 6 H 10.50; N 6.57. C12H23N02. Calculated %: С 67.57; H 10.87; N 6.57. The hydrochloride was obtained as colorless crystals with mp 206-207° and was soluble in water and alcohol. Found %: Cl 14.49; N 5.86. С12H23N02 • HCl. Calculated %: Cl 14.19; N 5.61.
The summary
An improved 3-hladiny method for the synthesis of 4-(carboxymethyl)-2,2,6,6-tetramethylpiperidine chloride 4 out of triacetonamine was developed. Total output of is ~66%. The probable mechanisms of reactions were discussed. The intermediate product 4-(ethoxycarbonylmethylene)-2,2,6,6-tetramethylpiperidine 2 and the target 4-(carboxymethyl)-2,2,6,6-tetramethylpiperidine chloride 4 characterized by refined melting points, TLC data and IR spectra and 1H NMR. Target 4-(carboxymethyl)-2,2,6,6-tetramethylpiperidine chloride 4 is a starting compound for the synthesis of useful nitroxyl radical containing a carboxyl group.
1H NMR 4-(carboxymethyl)-2,2,6,6-tetramethylpiperidine chloride
Дата добавления: 2015-05-30; просмотров: 782;
