Introduction – brief description of nitroxyl radicals
Synthesized 4-(carboxymethyl)-2,2,6,6-tetramethylpiperidine chloride is the source in the synthesis of nitroxyl radical containing a carboxyl group. Nitroxyl radical is a class of organic compounds containing the grouping 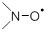 , which is part of the cycle and associated with two tertiary carbon atoms in most stable nitroxyl radicals. Examples of structures of nitroxyl radicals are given in figure 1.1.
, which is part of the cycle and associated with two tertiary carbon atoms in most stable nitroxyl radicals. Examples of structures of nitroxyl radicals are given in figure 1.1.
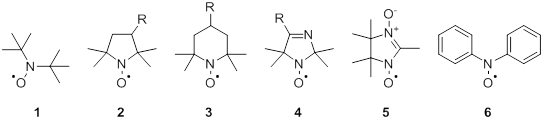
Figure 1.1 R-functional groups or complex fragments of molecules.
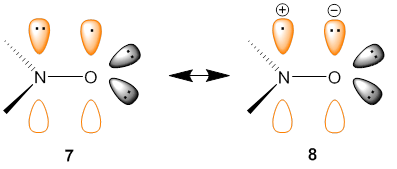
Electronic structure of nitroxyl group corresponds to the resonance structures 7 ↔ 8 [1].
The main factors of stability of di-tert-alkylnitroxyl radicals (NR) are: 1) energy stabilization of the N–O bond is ~127 kJ/mol [2]; 2) the inability of the disproportionation to nitrone and hydroxylamine; 3) very weak transfer of spin density beyond the tertiary C-atoms [3].
The using of NR based on their physical and chemical properties. It is paramagnetic, so NR could be used in researches by the electron paramagnetic resonance. And NR has unique oxidation-reduction properties like transition metal.
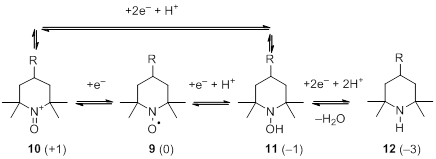 |
Figure 1.2 Oxidation-reduction reactions. There are N-oxidation state in brackets.
That is why NR are used as antioxidants, vasodilators, for radiation protection of organisms and so on.
4-(Carboxymethyl)-2,2,6,6-tetramethylpiperidine chloride 4 is a starting compound in the synthesis of relatively inaccessible valuable nitroxyl radical is 4-carboxymethyl-2,2,6,6-tetramethylpiperidine-1-oxil 5. Radical 5 was used, in particular, in the synthesis of nitroxyl derivatives of heparin, which are of interest as antioxidants and enhancers of contrast in NMR imaging [4; 5].
Дата добавления: 2015-05-30; просмотров: 1104;
