OCCURRENCE. Boron cannot be found naturally in the elemental form
Boron cannot be found naturally in the elemental form. However, it is found in the form of its various compounds. The most important compounds of boron are borates which contain the oxides of calcium and sodium.
USES
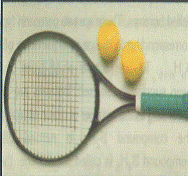
Boron is an additive in the manufacture of steel and lightweight alloys. Such alloys are strong and light and are ideal for use in tennis and badminton rackets. Alloy steel is used in the missile and rocket industries. Since boron absorbs the neutrons produced in nuclear reactions, it is used as control rods in nuclear power stations in the form of boron steel or in the form of B4C. A lightweight alloy of boron
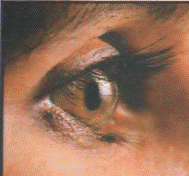
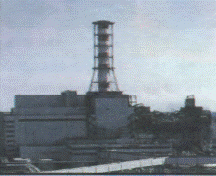
The isotope boron -10, is used as a moderator in control rods in nuclear reactors. Boron is a good absorber of neutrons. The Russians dumped quantities of boron on the stricken Chernobyl reactor. Amorphous boron is used in flares to give a green color when fired. Boron compounds are used in the manufacture of fiberglass insulation, textile products and ceramics, and eye disinfectant. When boron oxide is added to glass, boronsilicate glasses are produced. These glasses have a low rate of thermal expansion. They can be subjected to rapid heating and cooling without cracking. These glasses are used in Pyrex glassware, which is used in kitchens and laboratories. In agriculture, small amounts of boron, in the form of boric acid or borates, are necessary for the growth of plants, but in larger quantities the element may be harmful. Today, many scientists have ideas about boron and its compounds as an important source of energy in the future.
Дата добавления: 2015-02-05; просмотров: 772;
