The potentiometric measurement.
In potentiometry we measure the potential of an electrochemical cell under static conditions. Because no current—or only a negligible current—flows through the electrochemical cell, its composition remains unchanged. For this reason, potentiometry is a useful quantitative method. The first quantitative potentiometric applications appeared soon after the formulation, in 1889, of the Nernst equation, which relates an electrochemical cell’s potential to the concentration of electroactive species in the cell.
Potentiometry initially was restricted to redox equilibria at metallic electrodes, limiting its application to a few ions. In 1906, Cremer discovered that the potential difference across a thin glass membrane is a function of pH when opposite sides of the membrane are in contact with solutions containing different concentrations of H3O+. This discovery led to the development of the glass pH electrode in 1909. Other types of membranes also yield useful potentials. For example, in 1937 Kolthoff and Sanders showed that a pellet of AgCl can be used to determine the concentration of Ag+. Electrodes based on membrane potentials are called ion-selective electrodes, and their continued development extends potentiometry to a diverse array of analytes.
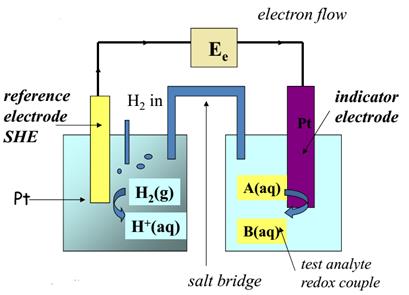
So in a potentiometric measurement two electrodes are used. These consist of the indicatoror sensing electrode, and a referenceelectrode. Electroanalytical measurements relating potential to analyte concentration rely on the response of one electrode only (the indicator electrode). The other electrode, the reference electrode is independent of the solution composition and provides a stable constant potential. The open circuit cell potential is measured using a potential measuring device such as a potentiometer, a high impedance voltameter or an electrometer.
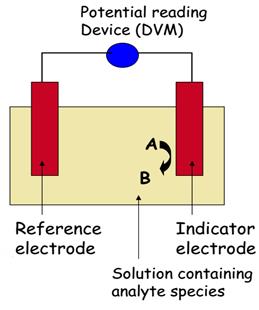
Figure 5 – Scheme of a potentiometric measurement
A schematic diagram of a typical potentiometric electrochemical cell is shown in Figure 6. The electrochemical cell consists of two half-cells, each containing an electrode immersed in a solution of ions whose activities determine the electrode’s potential. A SALT BRIDGE containing an inert electrolyte, such as KCl, connects the two half-cells. The ends of the salt bridge are fixed with porous frits, allowing the electrolyte’s ions to move freely between the half-cells and the salt bridge. This movement of ions in the salt bridge completes the electrical circuit.
By convention, we identify the electrode on the left as the ANODE and assign to it the oxidation reaction; thus
Zn (s) 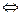 Zn2+ (aq) + 2e-
Zn2+ (aq) + 2e-
The electrode on the right is the CATHODE, where the reduction reaction occurs.
Ag + (aq) + e- 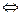 Ag (s)
Ag (s)
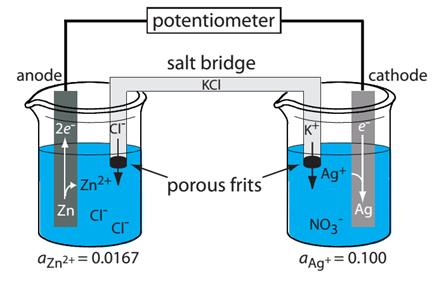
Figure 6 -Example of a potentiometric electrochemical cell. The activities of Zn2+ and Ag+ are shown below the two half-cells.
The potential of the electrochemical cell is for the reaction
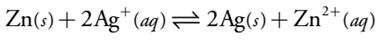
We also define potentiometric electrochemical cells such that the cathode is the indicator electrode and the anode is the reference electrode.
Instrumentation
Дата добавления: 2018-11-25; просмотров: 638;
