Controlling and Measuring Current and Potential
Electrochemical measurements are made in an electrochemical cell consisting of two or more electrodes and the electronic circuitry for controlling and measuring the current and the potential. In this section we introduce the basic components of electrochemical instrumentation.
The simplest electrochemical cell uses two electrodes. The potential of one electrode is sensitive to the analyte’s concentration, and is called the working electrode or the indicator electrode. The second electrode, which we call the counter electrode, completes the electrical circuit and provides a reference potential against which we measure the working electrode’s potential. Ideally the counter electrode’s potential remains constant so that we can assign to the working electrode any change in the overall cell potential. If the counter electrode’s potential is not constant, we replace it with two electrodes: a reference electrode whose potential remains constant and an auxiliary electrode that completes the electrical circuit.
Because we cannot simultaneously control the current and the potential, there are only three basic experimental designs: (1) we can measure the potential when the current is zero, (2) we can measure the potential while controlling the current, and (3) we can measure the current while controlling the potential. Each of these experimental designs relies on Ohm’s law, which states that a current, i, passing through an electrical circuit of resistance, R, generates a potential, E.
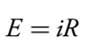
Each of these experimental designs uses a different type of instrument. To help us understand how we can control and measure current and potential, we will describe these instruments as if the analyst is operating them manually. To do so the analyst observes a change in the current or the potential and manually adjusts the instrument’s settings to maintain the desired experimental conditions. It is important to understand that modern electrochemical instruments provide an automated, electronic means for controlling and measuring current and potential, and that they do so by using very different electronic circuitry.
Potentiometry
Potentiometry is the field of electroanalytical chemistry in which potential is measured under the conditions of no current flow. The measured potential may then be used to determine the analytical quantity of interest, generally the concentration of some component of the analyte solution. The potential that develops in the electrochemical cell is the result of the free energy change that would occur if the chemical phenomena were to proceed until the equilibrium condition has been satisfied.
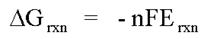
This concept is typically introduced in quantitative analysis courses in relation to electrochemical cells that contain an anode and a cathode. For these electrochemical cells, the potential difference between the cathode electrode potential and the anode electrode potential is the potential of the electrochemical cell.

If the reaction is conducted under standard state conditions, this equation allows the calculation of the standard cell potential. When the reaction conditions are not standard state, however, one must utilize the Nernst equation to determine the cell potential.
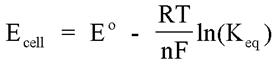
Physical phenomena which do not involve explicit redox reactions, but whose initial conditions have a non-zero free energy, also will generate a potential. An example of this would be ion concentration gradients across a semi-permeable membrane. This can also be a potentiometric phenomena, and is the basis of measurements that use ion-selective electrodes.
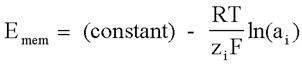
Дата добавления: 2018-11-25; просмотров: 506;
