OPTIMUM CONDITIONS OF PHOTOMETRIC DEFINITION
Components of a single-beam spectrophotometer
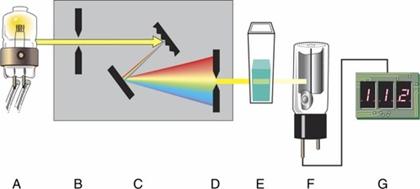
A, exciter lamp; B, entrance slit; C, monochromator; D, exit slit; E, cuvet; F, photodetector; G, LED display.
§ Molecular–absorption method is based on measurement of absorption by molecules (or ions) substances of electromagnetic radiation of an optical range:
§ Colorimetry in which visible light was absorbed by a sample. The concentration of analyte was determined visually by comparing the sample’s color to that of a set of standards using Nessler tubes (as described at the beginning of this chapter), or by using an instrument called a colorimeter.
§ Photocolorimetry - in which polychromatic light was absorbed by a sample
§ Spectrophotometry - in which monochromatic light was absorbed by a sample
§ UV - Spectrum (100-200 to 380-400 nanometers)
§ Visible spectrum (380-400 to 780-800 nanometers)
Instrumentation
Frequently an analyst must select, from several instruments of different design, the one instrument best suited for a particular analysis. In this section we examine some of the different types of instruments used for molecular absorption spectroscopy, emphasizing their advantages and limitations. Methods of sample introduction are also covered in this section.
Instrument Designs for Molecular UV/Vis Absorption
The simplest instrument currently used for molecular UV/Vis absorption is the filter photometershown in Figure, which uses an absorption or interference filter to isolate a band of radiation.

The filter is placed between the source and sample to prevent the sample from decomposing when exposed to high-energy radiation. A filter photometer has a single optical path between the source and detector and is called a single-beam instrument.
The instrument is calibrated to 0% T while using a shutter to block the source radiation from the detector. After removing the shutter, the instrument is calibrated to 100% T using an appropriate blank. The blank is then replaced with the sample, and its transmittance is measured. Since the source’s incident power and the sensitivity of the detector vary with wavelength, the photometer must be recalibrated whenever the filter is changed. In comparison with other spectroscopic instruments, photometers have the advantage of being relatively inexpensive, rugged, and easy to maintain. Another advantage of a photometer is its portability, making it a useful instrument for conducting spectroscopic analyses in the field. A disadvantage of a photometer is that it cannot be used to obtain an absorption spectrum.
Instruments using monochromators for wavelength selection are called spectrometers. In absorbance spectroscopy, where the transmittance is a ratio of two radiant powers, the instrument is called a spectrophotometer. The simplest spectrophotometeris a single-beam instrument equipped with a fixedwavelength monochromator, the block diagram for which is shown in Figure.

Photo courtesy of Fisher Scientific
Single-beam spectrophotometers are calibrated and used in the same manner as a photometer. One common example of a single-beam spectrophotometer is the Spectronic-20 manufactured by Milton-Roy. The Spectronic-20 can be used from 340 to 625 nm (950 nm with a red-sensitive detector), and has a fixed effective bandwidth of 20 nm. Because its effective bandwidth is fairly large, this instrument is more appropriate for a quantitative analysis than for a qualitative analysis. Battery-powered, hand-held single-beam spectrophotometers are available, which are easily transported and can be used for on-site analyses.
Other single-beam spectrophotometers are available with effective bandwidths of 2–8 nm. Fixed-wavelength single-beam spectrophotometers are not practical for recording spectra since manually adjusting the wavelength and recalibrating the spectrophotometer is awkward and time-consuming. In addition, the accuracy of a single-beam spectrophotometer is limited by the stability of its source and detector over time.
The limitations of fixed-wavelength, single-beam spectrophotometers are minimized by using the double-beam in-time spectrophotometer as shown in Figure.
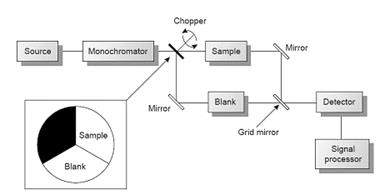
Block diagram for a double-beam in-time scanning spectrophotometer with photo of a typical instrument

A chopper, similar to that shown in the insert, controls the radiation’s path, alternating it between the sample, the blank, and a shutter. The signal processor uses the chopper’s known speed of rotation to resolve the signal reaching the detector into that due to the transmission of the blank (P0) and the sample (PT). By including an opaque surface as a shutter it is possible to continuously adjust the 0% T response of the detector.
The effective bandwidth of a double-beam spectrophotometer is controlled by means of adjustable slits at the entrance and exit of the monochromator. Effective bandwidths of between 0.2 nm and 3.0 nm are common. A scanning monochromator allows for the automated recording of spectra. Double-beam instruments are more versatile than single-beam instruments, being useful for both quantitative and qualitative analyses; they are, however, more expensive.
The instrument designs considered thus far use a single detector and can only monitor one wavelength at a time. A linear photodiode array consists of multiple detectors, or channels, allowing an entire spectrum to be recorded in as little as 0.1 s.
Source radiation passing through the sample is dispersed by a grating. The linear photodiode array is situated at the grating’s focal plane, with each diode recording the radiant power over a narrow range of wavelengths.
The sample compartment for the instruments in Figures above provides a light-tight environment that prevents the loss of radiation, as well as the addition of stray radiation. Samples are normally in the liquid or solution state and are placed in cells constructed with UV/Vis-transparent materials, such as quartz, glass, and plastic (Figure).

Typical cells used in UV/Vis spectroscopy
Quartz or fused-silica cells are required when working at wavelengths of less than 300 nm where other materials show a significant absorption. The most common cell has a pathlength of 1 cm, although cells with shorter (³ 1 mm) and longer pathlengths (£ 10 cm) are available. Cells with a longer pathlength are useful for the analysis of very dilute solutions or for gaseous samples. The highest quality cells are constructed in a rectangular shape, allowing the radiation to strike the cell at a 90° angle, where losses to reflection are minimal. These cells, which are usually available in matched pairs having identical optical properties, are the cells of choice for double-beam instruments. Cylindrical test tubes are often used as a sample cell for simple, single-beam instruments, although differences in the cell’s pathlength and optical properties add an additional source of error to the analysis.
In some circumstances it is desirable to monitor a system without physically removing a sample for analysis. This is often the case, for example, with the on-line monitoring of industrial production lines or waste lines, for physiological monitoring, and for monitoring environmental systems. With the use of a fiber-optic probe it is possible to analyze samples in situ. A simple example of a remote-sensing, fiber-optic probe is shown in Figure a and consists of two bundles of fiber-optic cable.
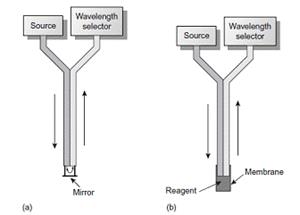
Example of fiber-optic probes
One bundle transmits radiation from the source to the sample cell, which is designed to allow for the easy flow of sample through the cell. Radiation from the source passes through the solution, where it is reflected back by a mirror. The second bundle of fiber-optic cable transmits the nonabsorbed radiation to the wavelength selector. In an alternative design (Figure b), the sample cell is a membrane containing a reagent phase capable of reacting with the analyte. When the analyte diffuses across the membrane, it reacts with the reagent phase, producing a product that absorbs UV or visible radiation. Nonabsorbed radiation from the source is reflected or scattered back to the detector. Fiber-optic probes that show chemical selectivity are called optrodes.
CLASSIFICATION OF OPTICAL METHODS OF THE ANALYSIS:
A major part of modern Instrumental Analytical Chemistry, focuses on the study of the energy exchange between electromagnetic radiation and matter. These interactions are visible to the naked eye, when the radiations concerned fall within the visible spectrum.
Дата добавления: 2018-11-25; просмотров: 691;
