Fundamental Limitations to Beers Law
Beer’s law is a limiting law that is valid only for low concentrations of analyte. There are two contributions to this fundamental limitation to Beer’s law. At higher concentrations the individual particles of analyte no longer behave independently of one another. The resulting interaction between particles of analyte may change the value of . A second contribution is that the absorptivity, a, and molar absorptivity, , depend on the sample’s refractive index. Since the refractive index varies with the analyte’s concentration, the values of a and will change. For sufficiently low concentrations of analyte, the refractive index remains essentially constant, and the calibration curve is linear.
Instrumental Limitations to Beer’s Law
There are two principal instrumental limitations to Beer’s law. The first limitation is that Beer’s law is strictly valid for purely monochromatic radiation; that is, for radiation consisting of only one wavelength.
However, even the best wavelength selector passes radiation with a small, but finite effective bandwidth. Using polychromatic radiation always gives a negative deviation from Beer’s law, but is minimized if the value of is essentially constant over the wavelength range passed by the wavelength selector.
For this reason, as shown in Figure 10.23, it is preferable to make absorbance measurements at a broad absorption peak.
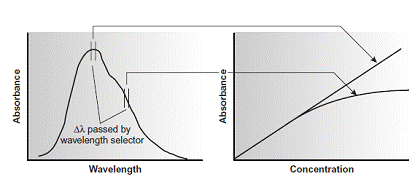
Effect of wavelength on the linearity of a Beer’s law calibration curve
In addition, deviations from Beer’s law are less serious if the effective bandwidth from the source is less than one tenth of the natural bandwidth of the absorbing species. When measurements must be made on a slope, linearity is improved by using a narrower effective bandwidth.
Stray radiationis the second contribution to instrumental deviations from Beer’s law. Stray radiation arises from imperfections within the wavelength selector that allows extraneous light to “leak” into the instrument. Stray radiation adds an additional contribution, Pstray, to the radiant power reaching the detector; thus 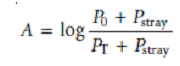
 For small concentrations of analyte, Pstray is significantly smaller than P0 and PT, and the absorbance is unaffected by the stray radiation. At higher concentrations of analyte, however, Pstray is no longer significantly smaller than PT and the absorbance is smaller than expected. The result is a negative deviation from Beer’s law.
For small concentrations of analyte, Pstray is significantly smaller than P0 and PT, and the absorbance is unaffected by the stray radiation. At higher concentrations of analyte, however, Pstray is no longer significantly smaller than PT and the absorbance is smaller than expected. The result is a negative deviation from Beer’s law.
Physical Limitations to Beer’s Law
NOT monochromaticity of light:
A = el×l×С.
NOT parallelism of light.
Temperature.
NOT identical value of refraction of solutions.
NOT proportionality of a photocurrent and intensity of a light
Chemical Limitations to Beer’s Law
Dilution of solution(than more of reagent excess, it is less deviation from the law);
рН of medium: state of metal ion
stability of complex ions
competitive reactions (for ligand)
competitive reactions (complexing agent)
polymerization and dissociation reactions
ox-red reactions
Chemical deviations from Beer’s law can occur when the absorbing species is involved in an equilibrium reaction. Consider, as an example, an analysis for the weak acid, HA. To construct a Beer’s law calibration curve, several standards containing known total concentrations of HA, Ctot, are prepared and the absorbance of each is measured at the same wavelength. Since HA is a weak acid, it exists in equilibrium with its conjugate weak base, A–
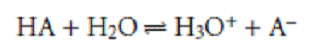
If both HA and A– absorb at the selected wavelength, then Beers law is written as
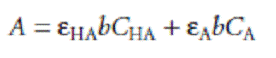
where CHA and CA are the equilibrium concentrations of HA and A–. Since the weak acid’s total concentration, Ctot, is
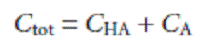
the concentrations of HA and A– can be written as
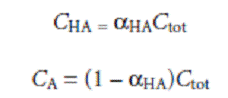
where aHA is the fraction of weak acid present as HA. Substituting equations gives
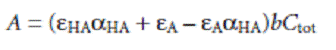
Because values of aHA may depend on the concentration of HA, equation may not be linear. A Beer’s law calibration curve of A versus Ctot will be linear if one of two conditions is met. If the wavelength is chosen such that eHA and eA are equal, then equation simplifies to
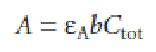
and a linear Beer’s law calibration curve is realized. Alternatively, if aHA is held constant for all standards, then equation will be a straight line at all wavelengths.
Because HA is a weak acid, values of aHA change with pH. To maintain a constant value for aHA, therefore, we need to buffer each standard solution to the same pH.
Depending on the relative values of eHA and eA, the calibration curve will show a positive or negative deviation from Beer’s law if the standards are not buffered to the same pH.
Дата добавления: 2018-11-25; просмотров: 1174;
