Bouguer-Lambert-Beer law
So:
The absorbance of a solution is proportional to concentration of light-absorbing substance and a thickness of a layer
Or
The relationship between a sample’s absorbance and the concentration of the absorbing species
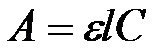
where: A – optical density (absorbance), ε – the molar absorptivity, C – concentration (molarity)
Additivity of optical densities
Beer’s law can be extended to samples containing several absorbing components provided that there are no interactions between the components. Individual absorbances, Ai, are additive. For a two-component mixture of X and Y, the total absorbance, Atot, is

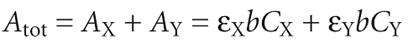
So
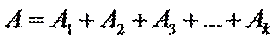

A = l(e1С1 + e2С2 + …ekСk)
| permeate | e (the molar absorptivity) |
| Iron (ІІІ) rhodanate | 103 |
| Complex Ti with H2O2 | 103 |
| Complex Ti with chromotrope acid | 105 |
| Complex Cu with ammonia | 5 ×102 |
| Complex Cu with dithizon | 5 ×104 |
| Complex Al with aluminon | 1,7 ×104 |
| Complex Al with 2-stilbazole | 3,5 ×104 |
Limitations to Beer’s Law
According to Beer’s law, a calibration curve of absorbance versus the concentration of analyte in a series of standard solutions should be a straight line with an intercept of 0 and a slope of ab or b. In many cases, however, calibration curves are found to be nonlinear (Figure 10.22).
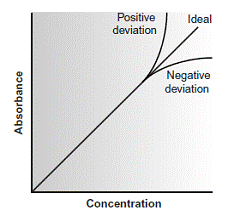
Calibration curves showing positive and negative deviations from Beer’s law.
Deviations from linearity are divided into three categories: fundamental, chemical, and instrumental.
Дата добавления: 2018-11-25; просмотров: 570;
