ALKALI METALS TO THE LEFT
Let's start on the left side of the periodic table. When looking for families, the first one you will find is the alkali metal family of elements. They are also known as the alkaline metals. You should remember that there is a separate group called the alkaline earth metals in Group Two. They are a very different family even though they have a similar name. That far left column is Group One (Group I). When we talk about the groups of the periodic table, scientists use Roman numerals when they write them out.
A FAMILY PORTRAIT
Who's in the family? Starting at the top we find hydrogen (H). But wait. That element is NOT in the family. When we told you about families, we said that they were groups of elements that react in similar ways. Hydrogen is a very special element of the periodic table and doesn't belong to any family. While hydrogen sits in Group I, it is NOT an alkali metal.
FAMILY BONDING
Now that we've covered that exception, the members of the family include: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs) and Francium (Fr). As with all families, these elements share traits. They are very reactive. Why? They all have one electron in their outer shell. That's one electron away from being happy (full shells). When you are that close to having a full shell, you want to bond with other elements and lose that electron. An increased desire to bond means you are more reactive. In fact, when you put some of these pure elements in water, they will cause huge explosions.
The alkali metals are also metals. That seems obvious from the name. Often, in chemistry, characteristics are given by the way elements look. You will find that the alkali group is shiny and light in weight. Their light weight and physical properties separate them from other metals. Alkali metals are not the type of metals you would use for coins or houses.
9.Alkaline earth metals
 HEADING TO GROUP TWO
HEADING TO GROUP TWO
So we just covered the alkali metals in Group I. You will find the alkaline earth metals right next door in Group II. This is the second most reactive family of elements in the periodic table. Did you know why they are called alkaline? When these compounds are mixed in solutions, they are likely to form solutions with a pH greater than 7. Those pH levels are defined as 'basic' or 'alkaline' solutions.
A FAMILY PORTRAIT
Who's in the family? The members of the alkaline earth metals include: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra). As with all families, these elements share traits. While not as reactive as the alkali metals, this family knows how to make bonds very easily. Each of them has two electrons in their outer shells. They are ready to give up those two electrons in electrovalent bonds. Sometimes you will see them with two halogen atoms (BeF2) and sometimes they might form a double bond (CaO). It's all about giving up those electrons to have a full outer shell.
As you get to the bottom of the list, you will find the radioactive radium (Ra). While radium is not found around your house anymore, it was used in glow-in-the-dark paints. The other elements are found in many items including fireworks, batteries, flashbulbs, and special alloys. The lighter alkaline earth metals such as magnesium and calcium are very important in animal and plant physiology. You all know that calcium helps build your bones.
 10. Transition metals
10. Transition metals
TRANSITIONING
Lets start off by telling you that there are a lot of elements that are considered transition metals. Which metals are the transition metals?
21 (Scandium) through 29 (Copper)
39 (Yttrium) through 47 (Silver)
57 (Lanthanum) through 79 (Gold)
89 (Actinium) and all higher numbers.
 WHAT MAKES THEM SO SPECIAL?
WHAT MAKES THEM SO SPECIAL?
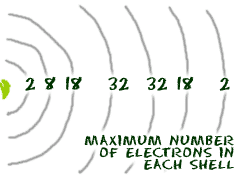
It all has to do with their shells/orbitals. In our chemistry course we try to stick to the first 18 elements because they are easy to explain. Transition metals are good examples of advanced shell ideas. They have a lot of electrons and distribute them in different ways.
Transition metals are able to put more than eight electrons in the shell that is one in from the outermost shell. Think about argon (Ar). It has 18 electrons set up in a 2-8-8 order. Scandium is only 3 spots away with 21 electrons, but it has a configuration of 2-8-9-2. Wow! This is where it starts. This is the point in the periodic table where you can place more than 8 electrons in a shell.
The transition metals are able to put up to 32 electrons in their second to last shell. Something like gold (Au) has an organization of 2-8-18-32-18-1. Of course, there are still some rules. No shell can have more than 32 electrons. It's usually 18 or 32 for the maximum number of electrons.
ONE MORE THING
Most elements can only use electrons from their outer orbital to bond with other elements. Transition metals can use the two outermost shells/orbitals to bond with other elements. It's a chemical trait that allows them to bond with many elements in a variety of shapes. Why can they do that?
As you learn more, you will discover that most transition elements actually have two shells that are not happy. Whenever you have a shell that is not happy, its electrons can bond with other elements. Example: Molybdenum (Mo) with 42 electrons. The configuration is 2-8-18-13-1. The shells with 13 and 1 are not happy. Those two orbitals can use the electrons to bond with other atoms.
LANTHANIDE
Дата добавления: 2016-01-11; просмотров: 841;
