FAMILIES STICK TOGETHER
Overview
 The Same Everywhere
The Same Everywhere
Now we're getting to the heart and soul of the way your universe works. Elements are the building blocks of all matter. As far as we know, there are so many basic elements. Up to this point in time we have discovered/created over 100. While there may be more out there to discover, the basic elements remain the same. Iron (Fe) atoms found on Earth are identical to iron atoms found on meteorites. The iron atoms on Mars that make the soil red are the same too.
The point is... With the tools you learn here, you can explore and understand the universe. You will never stop discovering new reactions and compounds, but the elements will remain the same.
2. Periodic Table and the Elements
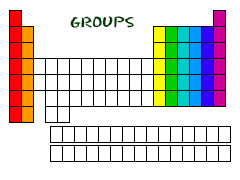 Elements as Building Blocks
Elements as Building Blocks
As you probably saw, the periodic table is organized like a big grid. The elements are placed in specific places because of the way they look and act. If you have ever looked at a grid, you know that there are rows (left to right) and columns (up and down). The periodic table has rows and columns, too, and they each mean something different.
You've got Your Periods...
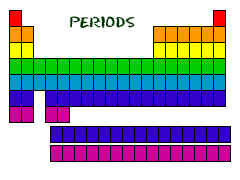 Even though they skip some squares in between, all of the rows go left to right. When you look at a periodic table, each of the rows is considered to be a different period (Get it? Like PERIODic table.). In the periodic table, elements have something in common if they are in the same row. All of the elements in a period have the same number of atomic orbitals. Every element in the top row (the first period) has one orbital for its electrons. All of the elements in the second row (the second period) have two orbitals for their electrons. It goes down the periodic table like that. At this time, the maximum number of electron orbitals or electron shells for any element is seven.
Even though they skip some squares in between, all of the rows go left to right. When you look at a periodic table, each of the rows is considered to be a different period (Get it? Like PERIODic table.). In the periodic table, elements have something in common if they are in the same row. All of the elements in a period have the same number of atomic orbitals. Every element in the top row (the first period) has one orbital for its electrons. All of the elements in the second row (the second period) have two orbitals for their electrons. It goes down the periodic table like that. At this time, the maximum number of electron orbitals or electron shells for any element is seven.
And Your Groups
Now you know about periods. The periodic table has a special name for its columns, too. When a column goes from top to bottom, it's called a group. The elements in a group have the same number of electrons in their outer orbital. Every element in the first column (group one) has one electron in its outer shell. Every element on the second column (group two) has two electrons in the outer shell. As you keep counting the columns, you'll know how many electrons are in the outer shell. There are some exceptions to the order when you look at the transition elements, but you get the general idea.
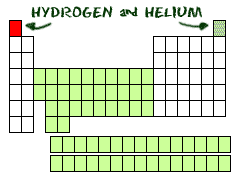 Two at the Top
Two at the Top
Hydrogen (H) and helium (He) are special elements. Hydrogen can have the talents and electrons of two groups, one and seven. To scientists, hydrogen is sometimes missing an electron, and sometimes it has an extra. Helium is different from all of the other elements. It can only have two electrons in its outer shell. Even though it only has two, it is still grouped with elements that have eight (inert gases).
The elements in the center section are called transition elements. They have special electron rules.
Element List
We've got 18 to choose from. From the beginning we've been asked, "Why only cover 18?" The rules for the first 18 elements are very straight-forward. (1) Electrons fit nicely into three shells. (2) These elements make up most of the matter in the universe. (3) It's a lot easier to remember facts about 18 elements than over 100 elements.
THE LIST: Element 1: Hydrogen, Element 2: Helium, Element 3: Lithium, Element 4: Beryllium, Element 5: Boron, Element 6: Carbon,
Element 7: Nitrogen, Element 8: Oxygen, Element 9: Fluorine, Element 10: Neon, Element 11: Sodium, Element 12: Magnesium,
Element 13: Aluminum, Element 14: Silicon, Element 15: Phosphorus,
Element 16: Sulfur, Element 17: Chlorine, Element 18: Argon.
MORE THAN 18? Yes, it's true. After these many years, we have added elements 18-36 to our list of elements. This next set of elements is from the fourth period/row of the table. Element 19: Potassium, Element 20: Calcium, Element 21: Scandium, Element 22: Titanium, Element 23: Vanadium, Element 24: Chromium, Element 25: Manganese, Element 26: Iron, Element 27: Cobalt, Element 28: Nickel, Element 29: Copper, Element 30: Zinc, Element 31: Gallium, Element 32: Germanium, Element 33: Arsenic, Element 34: Selenium, Element 35: Bromine, Element 36: Krypton.
Families
FAMILIES STICK TOGETHER
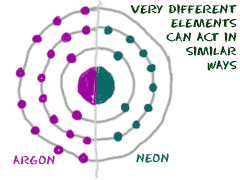 We just covered the columns and rows of the periodic table. There are also other, less specific, groups of elements. These groups are all over the table. Scientists group these families of elements by their chemical properties. Each family reacts a different way with the outside world. Metals behave differently than gases and there are even different types of metals. Some don't react, others are very reactive, and some are metallic.
We just covered the columns and rows of the periodic table. There are also other, less specific, groups of elements. These groups are all over the table. Scientists group these families of elements by their chemical properties. Each family reacts a different way with the outside world. Metals behave differently than gases and there are even different types of metals. Some don't react, others are very reactive, and some are metallic.
Usually, the columns of the periodic table are used to define families. The inert gases are all located in the far right column of the table. That column is labeled Group Zero. The other possibility that can happen are elements in a series. Good examples of a series of elements in the same family are the transition metals.
The thing to remember is... A family of elements can be found in several ways. You need to run tests and study the elements to determine their properties. Only after that testing, you can determine what family an element belongs to.
EXAMPLES OF FAMILIES:- Alkali Metals; - Alkaline Earth Metals; - Transition Metals; - Halogen Gases; - Inert Gases (Noble Gases)
EXAMPLES OF PHYSICAL PROPERTIES:- Density; - Boiling Point; - Melting Point; - Conductivity; - Heat Capacity
EXAMPLES OF CHEMICAL PROPERTIES:- Valence; - Reactivity; - Radioactivity.
HALOGENS
Дата добавления: 2016-01-11; просмотров: 1016;
