Matter and it¢s structure
Most of the Universe consists of matter and energy. Energy is the capacity to do work. Matter has mass and occupies space. All matter is composed of basic elements that cannot be broken down to substances with different chemical or physical properties.
Elements are substances consisting of one type of atom, for example Carbon atoms make up diamond, and also graphite. Pure (24K) gold is composed of only one type of atom, gold atoms. Atoms are the smallest particle into which an element can be divided. The ancient Greek philosophers developed the concept of the atom, although they considered it the fundamental particle that could not be broken down. Since the work of Enrico Fermi and his colleagues, we now know that the atom is divisible, often releasing tremendous energies as in nuclear explosions or (in a controlled fashion in) thermonuclear power plants.
Subatomic particles were discovered during the 1800s. For our purposes we will concentrate only on three of them, summarized in Table 1. The proton is located in the center (or nucleus) of an atom, each atom has at least one proton. Protons have a charge of +1, and a mass of approximately 1 atomic mass unit (amu). Elements differ from each other in the number of protons they have, e.g. Hydrogen has 1 proton; Helium has 2.
The neutron also is located in the atomic nucleus (except in Hydrogen). The neutron has no charge, and a mass of slightly over 1 amu. Some scientists propose the neutron is made up of a proton and electron-like particle.
The electron is a very small particle located outside the nucleus. Because they move at speeds near the speed of light the precise location of electrons is hard to pin down. Electrons occupy orbitals, or areas where they have a high statistical probability of occurring. The charge on an electron is -1. Its mass is negligible (approximately 1800 electrons are needed to equal the mass of one proton).
Table 1. Subatomic particles of use in biology.
| Name | Symbol | Charge | Location | Mass |
| Proton | p+ | +1 | atomic nucleus | 1.6726 X 10-27 kg |
| Neutron | n, n0 | atomic nucleus | 1.6750 X 10-27 kg | |
| Electron | e− | -1 | electron orbital | 9.1095 X 10-31 kg |
The atomic number is the number of protons an atom has. It is characteristic and unique for each element. The atomic mass (also referred to as the atomic weight) is the number of protons and neutrons in an atom.
There is an easy way to represent isotopes using the atomic symbols. We use the construction
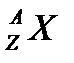
where X is the symbol of the element, A is the mass number, and Z is the atomic number. Thus, for the isotope of carbon that has 6 protons and 6 neutrons, the symbol is
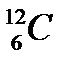
where C is the symbol for the element, 6 represents the atomic number, and 12 represents the mass number.
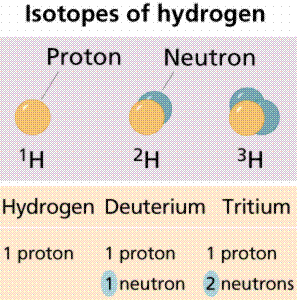 Atoms of the same element can have different numbers of neutrons, however. Atoms of the same element (i.e., atoms with the same number of protons) with different numbers of neutrons are called isotopes. Most naturally occurring elements exist as isotopes. For example, most hydrogen atoms have a single proton in their nucleus. However, a small number (about one in a million) of hydrogen atoms have a proton and a neutron in their nuclei. This particular isotope of hydrogen is called deuterium. A very rare form of hydrogen has one proton and two neutrons in the nucleus; this isotope of hydrogen is called tritium. The sum of the number of protons and neutrons in the nucleus is called the mass number of the isotope.
Atoms of the same element can have different numbers of neutrons, however. Atoms of the same element (i.e., atoms with the same number of protons) with different numbers of neutrons are called isotopes. Most naturally occurring elements exist as isotopes. For example, most hydrogen atoms have a single proton in their nucleus. However, a small number (about one in a million) of hydrogen atoms have a proton and a neutron in their nuclei. This particular isotope of hydrogen is called deuterium. A very rare form of hydrogen has one proton and two neutrons in the nucleus; this isotope of hydrogen is called tritium. The sum of the number of protons and neutrons in the nucleus is called the mass number of the isotope.
Some isotopes are radioisotopes, which spontaneously decay, releasing radioactivity. Other isotopes are stable. Examples of radioisotopes are Carbon-14 (symbol 14C), and deuterium (also known as Hydrogen-2; 2H). Stable isotopes are 12C and 1H.
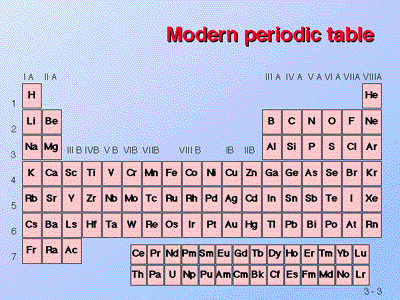
The elements are grouped together in a special chart called the periodic table. A simple periodic table is shown in Figure 3. The elements on the periodic table are listed in order of ascending atomic number. The periodic table has a special shape that will become important to us when we consider the organization of electrons in atom. One immediate use of the periodic table helps us identify metals and nonmetals. Nonmetals are in the upper right corner of the periodic table, on one side of the heavy line splitting the right-hand part of the chart. All other elements are metals.
| Figure 3. |
|
Дата добавления: 2018-11-25; просмотров: 496;
