CONJUGATIVE PROTEINS
Conjugated proteinsare two-component proteins which consist of simple protein and non-protein component (prosthetic group).
1. Chromoproteins (from Gr. chroma - color) consist of simple protein and the pigmented non-protein component bound to it. We distinguish hemoproteins and flavoproteins. They participate in such processes as breath, transport of oxygen and carbon dioxide, redox reactions, light- and colour perception, etc.
The group of hemoproteins include hemoglobin, myoglobin, cytochromes, catalase, peroxidases. All of them contain ferriporphyrin, but vary in protein structure, and carry out various biological functions. Specific distinctions of hemoglobin are caused by globin.
Let's consider hemoglobin structure. It is a blood protein. Non-protein component of hemoglobin is the heme (fig. 5). It’s a pigment giving blood its red color. The basis of its structure is the protoporphyrin IX. In the heme centre the atom of iron is bounded to two atoms of nitrogen covalently and with two others by coordination bonds.
A heme is "wrapped up" by one polypeptide chain. In a molecule of hemoglobin of an adult person НbА there are four polypeptide chains which together form the protein part of a molecule - globin. Two α-chains contain 141 amino-acid residues, two β-chains – 146 (fig. 6).
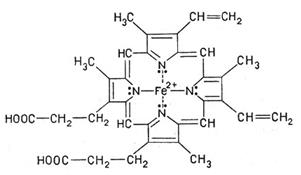

Fig. 5. Heme Fig. 6. Hemoglobin
In blood of an adult person there is also hemoglobin НbА2 (2α, 2δ chains, 2,5%) and НbA3 (less than 1%, differs by structure of b-chains).
There is fetal hemoglobin HbF, consisting of 2 α- and 2 γ-chains. Hemoglobin F possesses the increased affinity to oxygen and allows rather small volume of blood of a fetus to carry out oxygen-bearing functions more effectively. Blood of the newborn contains up to 80% HbF, by the end of the 1st year of life it is almost entirely changed to НbА.
Diseases of hemoglobins (more than 200) are called hemoglobinoses.
1.Hemoglobinopathy, at the basis of which hereditary structural change of any chain of normal hemoglobin. In blood of a human about 150 various types of mutant hemoglobins are found.
Abnormal hemoglobins differ in physical and chemical properties (electrophoretic mobility, solubility, isoelectric point, affinity to oxygen).
Classical example of hemoglobinopathy is sickle-cell anemia. It widely spread in the countries of South America, Africa and South East Asia. Chemical defect is reduced to glutamic acid changing in 6th position from the N-end to valine in β-chains of a molecule of hemoglobin (HbS). It is the result of a mutation in DNA molecule. The HbS solubility and affinity to oxygen are reduced. Erythrocytes in the conditions of low partial pressure of oxygen take the form of a sickle. HbS after oxygen return in tissues turns in low solubility desoxiform and drops out into sediment in the form of spindle-shaped crystals. They distort a cell and lead to a hemolysis. The heterozygous form of anomaly proceeds asymptomatically or is accompanied by an easy hemolytic anemia. Homozygous individuals from the first months of life have the heavy form of sickle-cell anemia. Disease proceeds sharply, and children often die in an early age.
2. Thalassemia is a group of diseases with hereditary infringement of synthesis of one of globin chains. We distinguish α- and β-thalassemia. Hemoglobinopathy Н is one of the variants of a-thalassemia. It manifests in hemolytic anemia, the precipitation of hemoglobin H, enlarged spleen, severe osteal changes.
3. Iron-deficient anemia is infringement of synthesis of hemoglobin owing to deficiency of iron. Principal causes are blood loss and lack of nutrition rich with heme - meat and fish.
Дата добавления: 2018-09-24; просмотров: 406;
